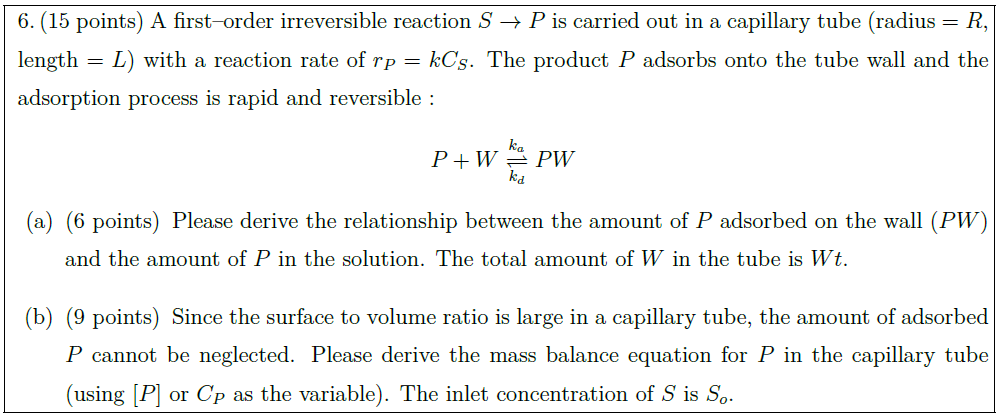
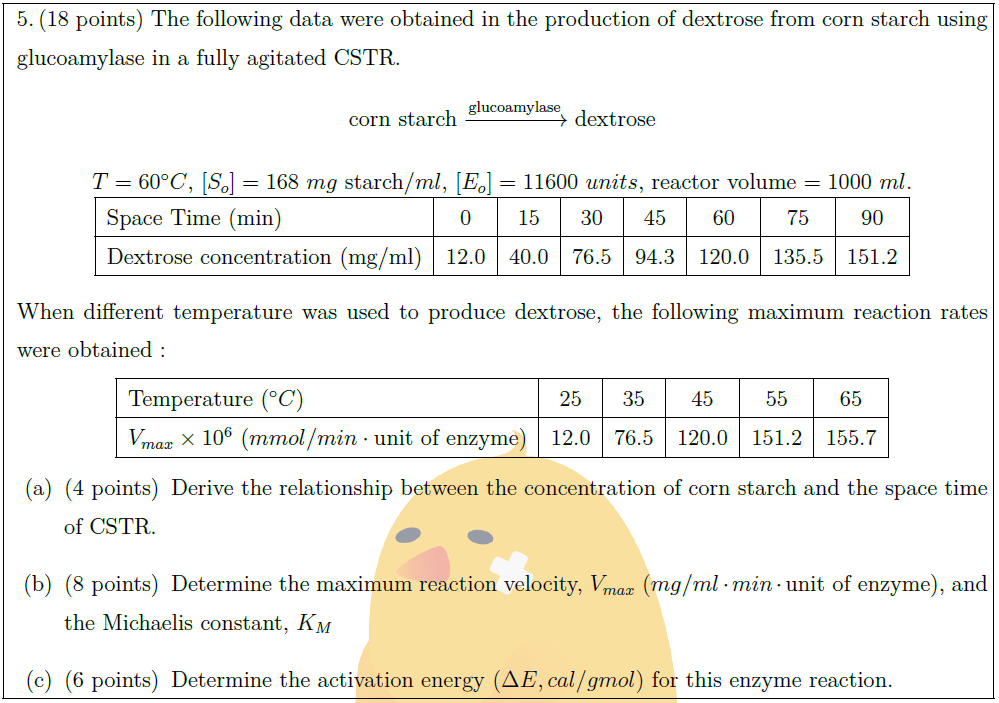
Solution:
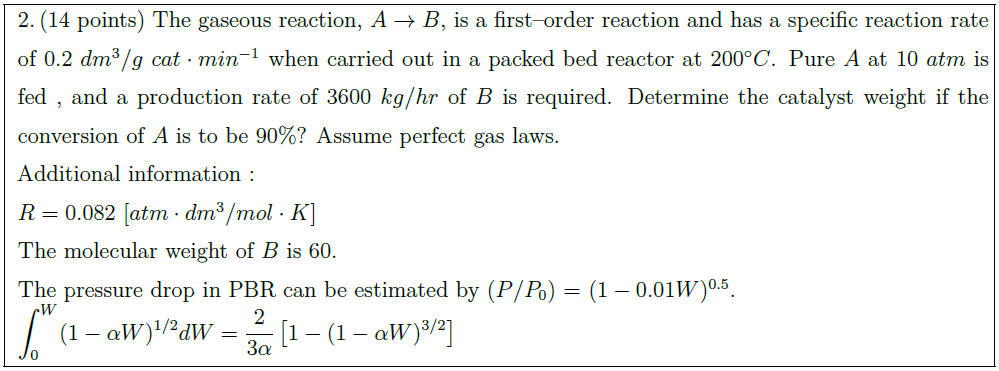
The gaseous reaction, $A \to B$, is a first–order reaction and has a specific reaction rate of $0.2\ dm^3/g\ cat \cdot min^{-1}$ when carried out in a packed bed reactor at $200^\circ C$. Pure $A$ at $10\ atm$ is fed , and a production rate of $3600\ kg/hr$ of $B$ is required. Determine the catalyst weight if the conversion of $A$ is to be $90 \%$? Assume perfect gas laws.\\
Additional information :\\
$R = 0.082\ [atm \cdot dm^3/ mol \cdot K]$\\
The molecular weight of $B$ is 60.\\
The pressure drop in PBR can be estimated by ($P/P_0$) $=$ ($1 – 0.01 W$)$^{0.5}$.\\
$\displaystyle \int_0^W (1 – \alpha W)^{1/2} dW = \frac{2}{3 \alpha} \left[ 1 – (1 – \alpha W)^{3/2} \right]$