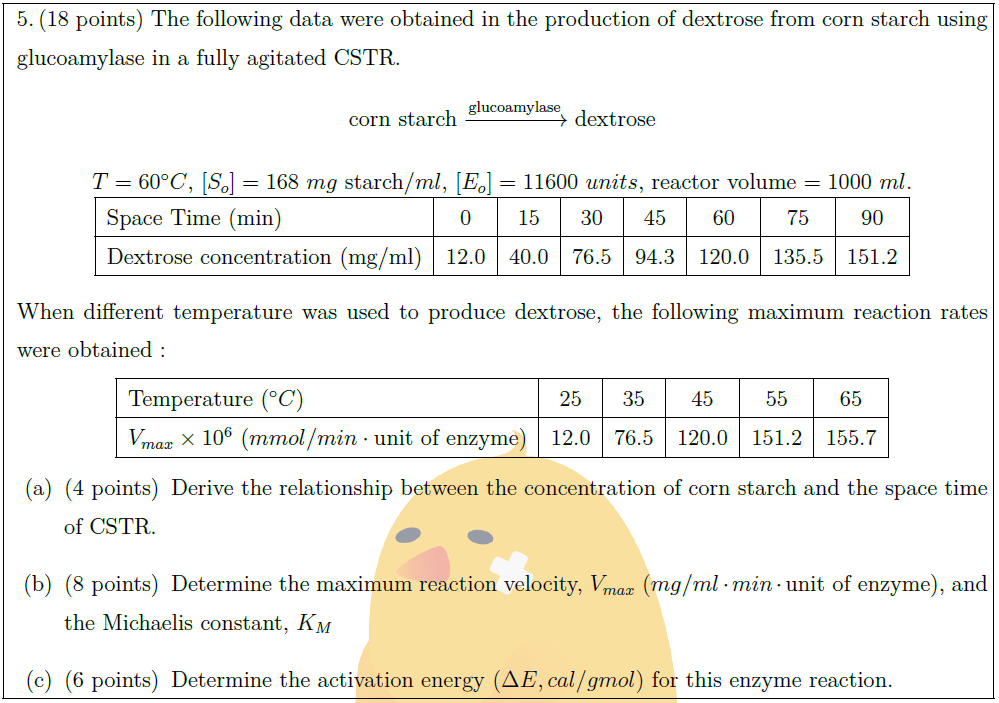
Solution:
Titanium nitride ($TiN$) films are used in decorative coatings as well as in wear–resistant tools. There is increasing interest in $TiN$ because of its thermal stability, good diffusion barrier properties, and its low electrical resistivity. Titanium nitride films were formed by $CVD$ from a mixture of $TiCl_4$, $NH_3$, $H_2$, and $Ar$. The following observations can be made from the experiment :\vspace*{-0.8em}\\
$\cdot$ \textbf{Observation 1 :} The rate of deposition is independent of $Ar$ and $H_2$.\\
$\cdot$ \textbf{Observation 2 :} At low partial pressures of both $TiCl_4$ and $NH_3$, the deposition rate\\
\hspace*{0.8em}appears to be first–order in $TiCl_4$ and second–order in $NH_3$.\\
$\cdot$ \textbf{Observation 3 :} At high partial pressures of $NH_3$, the rate varies inversely with $TiCl_4$.\vspace*{-0.8em}\\
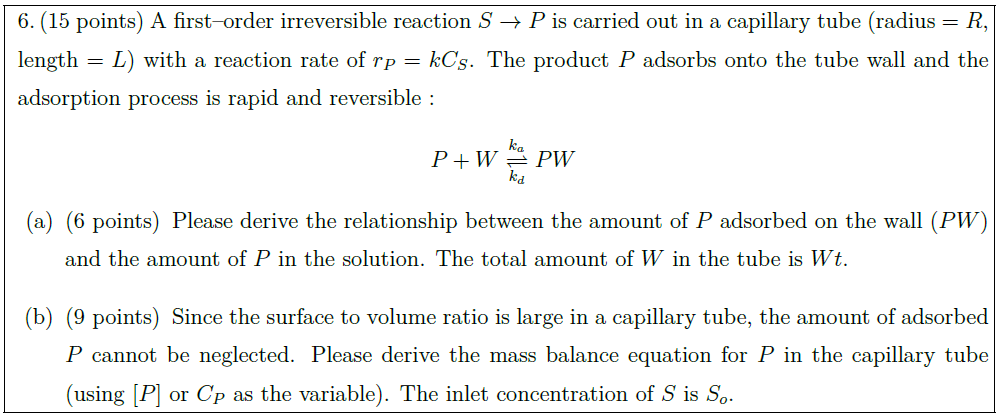
The following mechanism has been suggested for the reaction :\vspace*{-0.8em}
\begin{align*}
& TiCl_4 + 2 NH_3 \longleftrightarrow TiCl_4(NH_3)_2\quad \mbox{gas phase}\\
& TiCl_4(NH_3)_2 + S \longleftrightarrow TiCl_4(NH_3)_2 \cdot S\\
& TiCl_4(NH_3)_2 \cdot S + S \longrightarrow TiN + S + \mbox{gas products}\ (HCl, H_2, \mbox{etc.})
\end{align*}\vspace*{-0.5em}
It is believed that the gas–phase reaction to form the complex $TiCl_4(NH_3)_2$ is in equilibrium.\vspace*{0.5em}
\begin{parts}
\part [8] Determine the rate expression for the suggested mechanism.
\part [5] Evaluate if the rate expression obtained in (a) agree with each experimental observation?
\part [5] Determine the reaction rate parameters from the data given below.
\end{parts}
\begin{center}
\begin{tabular}{@{}lccccccccccccccc@{}}
\hline
$r_{Dep} \times 10^8$ & & 15 & & & 10 & & & 6 & & & 8.5 & & & 16 & \\
($mol\ TiN/cm^2 \cdot min$) & & & & & & & & & & & & & & & \\
\hline
$P_{NH_3}\ (mT)$ & & 79 & & & 79 & & & 79 & & & 60 & & & 100 & \\
$P_{TiCl_4}\ (mT)$ & & 1 & & & 3 & & & 10 & & & 2.3 & & & 2.3 & \\
\hline
\end{tabular}
\end{center}