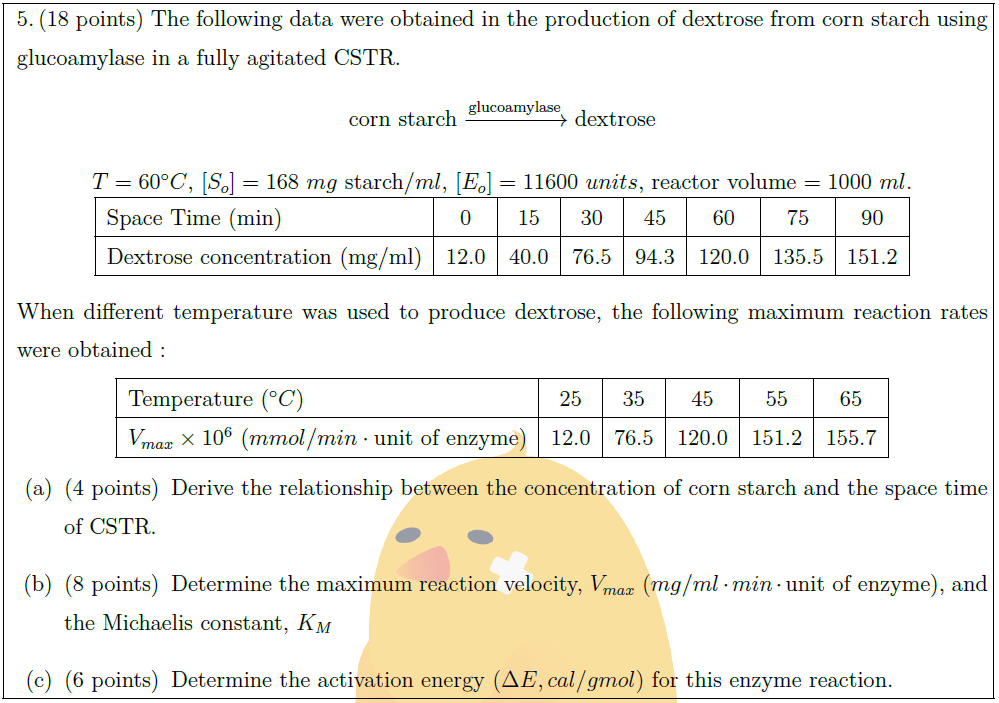
Solution:
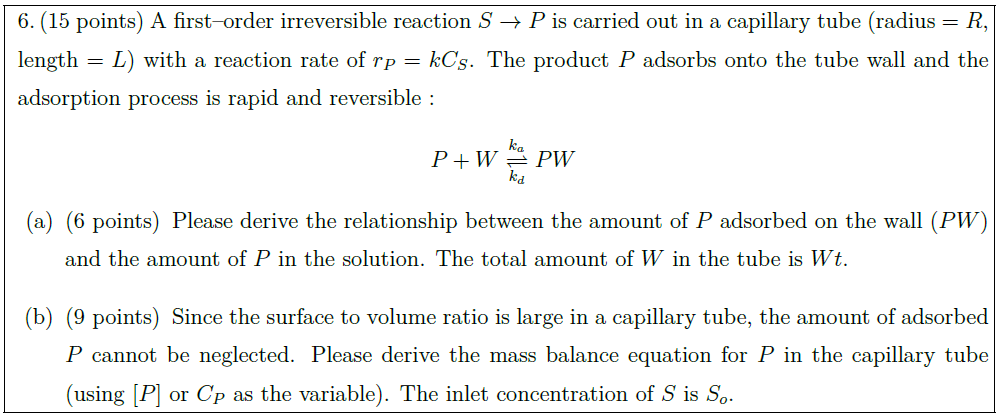
A first–order irreversible reaction $S \to P$ is carried out in a capillary tube (radius $= R$, length $= L$) with a reaction rate of $r_P = k C_S$. The product $P$ adsorbs onto the tube wall and the adsorption process is rapid and reversible :
\begin{align*}
P + W \underset{k_d}{\overset{k_a}{\rightleftharpoons}} PW
\end{align*}
\begin{parts}
\part [6] Please derive the relationship between the amount of $P$ adsorbed on the wall ($PW$) and the amount of $P$ in the solution. The total amount of $W$ in the tube is $Wt$.
\part [9] Since the surface to volume ratio is large in a capillary tube, the amount of adsorbed $P$ cannot be neglected. Please derive the mass balance equation for $P$ in the capillary tube (using $[P]$ or $C_P$ as the variable). The inlet concentration of $S$ is $S_o$.
\end{parts}