Solution:
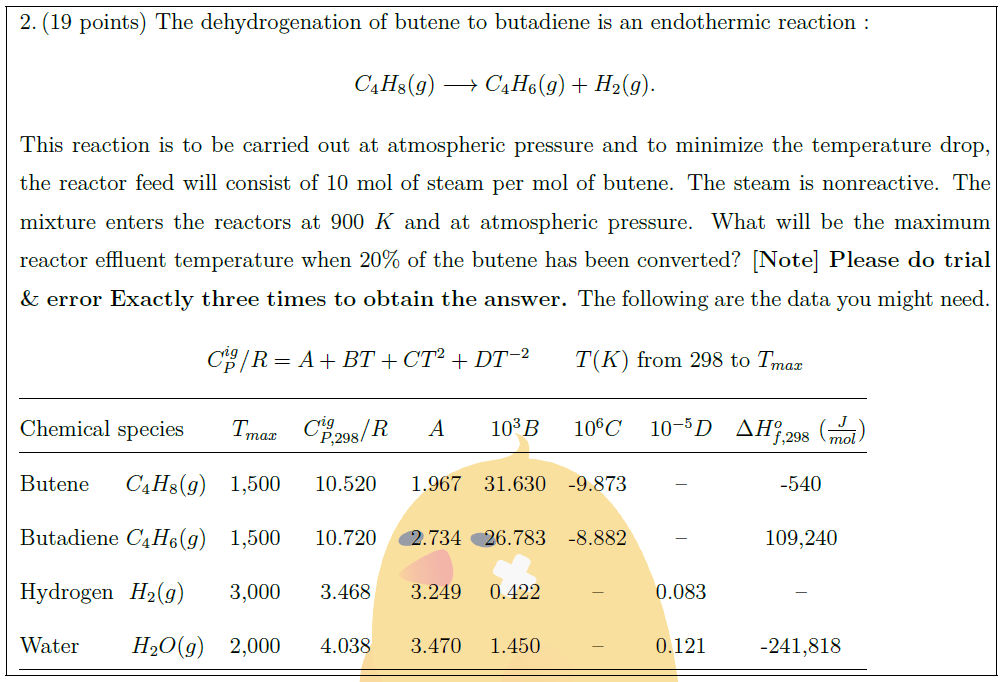
The dehydrogenation of butene to butadiene is an endothermic reaction :
\begin{align*}
C_4 H_8 (g) \longrightarrow C_4 H_6 (g) + H_2 (g).
\end{align*}
This reaction is to be carried out at atmospheric pressure and to minimize the temperature drop, the reactor feed will consist of 10 mol of steam per mol of butene. The steam is nonreactive. The mixture enters the reactors at $900\ K$ and at atmospheric pressure. What will be the maximum reactor effluent temperature when $20\%$ of the butene has been converted? {\bf [Note] Please do trial \& error Exactly three times to obtain the answer.} The following are the data you might need.
$$C_P^{ig} / R = A + BT + CT^2 + DT^{-2}\quad \quad T(K)\ \mbox{from}\ 298\ \mbox{to}\ T_{max}$$
\begin{tabular}{@{}lccccccc@{}}
\hline
Chemical species & $T_{max}$ & $C_{P, 298}^{ig} / R$ & $A$ & $10^3 B$ & $10^6 C$ & $10^{-5} D$ & $\Delta H_{f, 298}^{o}\ (\frac{J}{mol})$\\
\hline
Butene\ \ \ \ \ $C_4 H_8 (g)$ & 1,500 & 10.520 & 1.967 & 31.630 & -9.873 & — & -540\\
Butadiene $C_4 H_6 (g)$ & 1,500 & 10.720 & 2.734 & 26.783 & -8.882 & — & 109,240\\
Hydrogen\ \ $H_2 (g)$ & 3,000 & 3.468 & 3.249 & 0.422 & — & 0.083 & –\\
Water\ \ \ \ \ \ \ $H_2 O (g)$ & 2,000 & 4.038 & 3.470 & 1.450 & — & 0.121 & -241,818\\
\hline
\end{tabular}