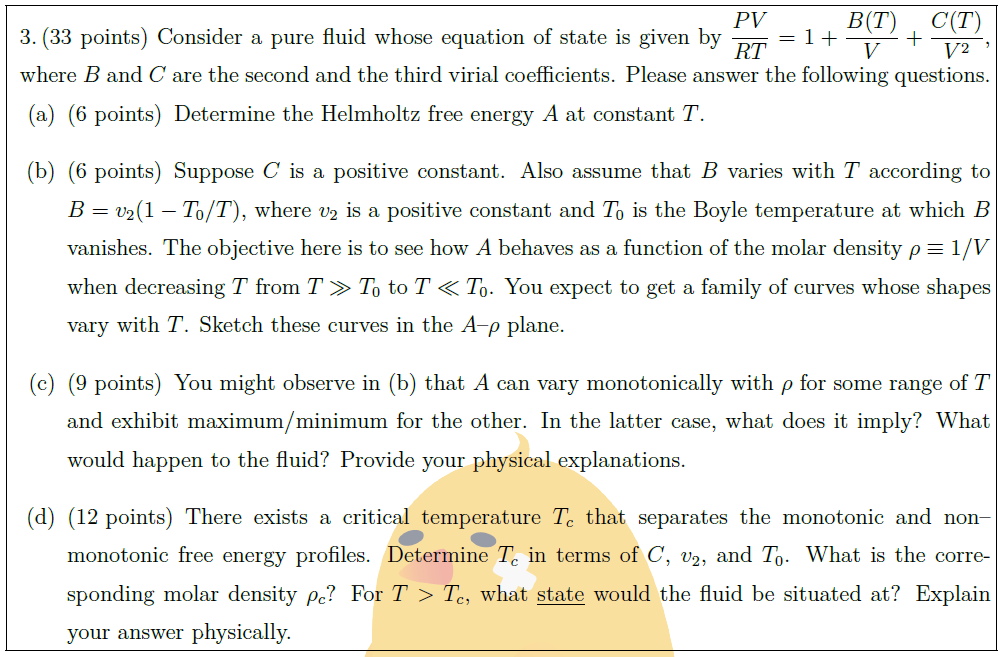
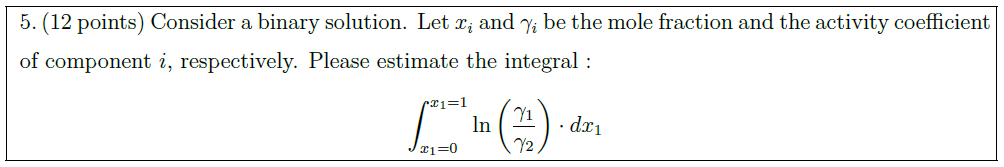Solution:
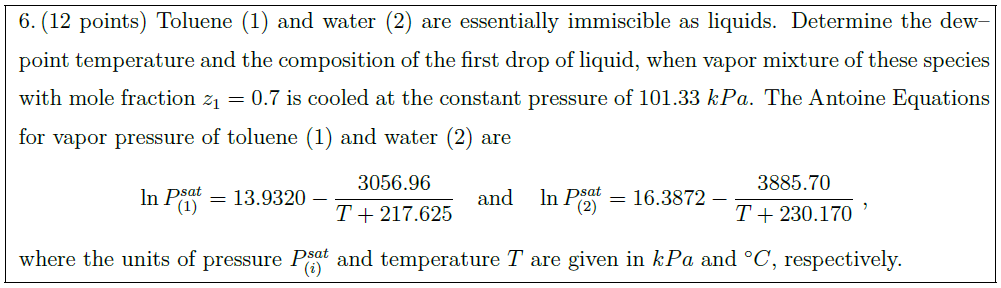
Toluene (1) and water (2) are essentially immiscible as liquids. Determine the dew–point temperature and the composition of the first drop of liquid, when vapor mixture of these species with mole fraction $z_1 = 0.7$ is cooled at the constant pressure of $101.33\ kPa$. The Antoine Equations for vapor pressure of toluene (1) and water (2) are
\begin{align*}
\ln P_{(1)}^{sat} = 13.9320 – \frac{3056.96}{T + 217.625}\quad \mbox{and}\quad \ln P_{(2)}^{sat} = 16.3872 – \frac{3885.70}{T + 230.170}\ ,
\end{align*}
where the units of pressure $P_{(i)}^{sat}$ and temperature $T$ are given in $kPa$ and $^\circ C$, respectively.