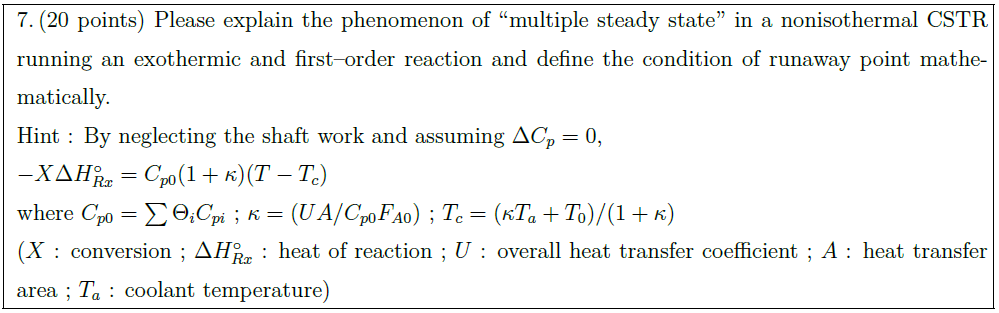
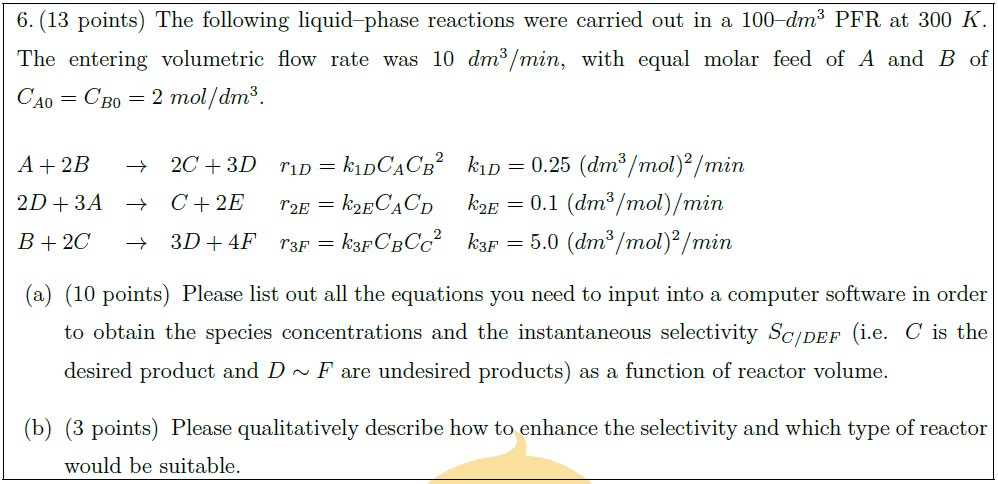
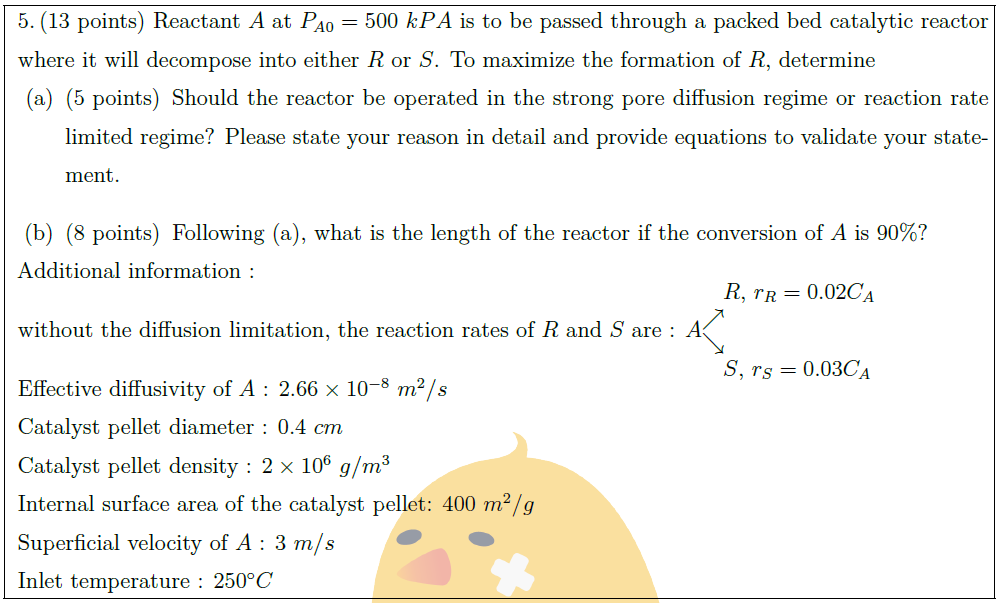
Solution:
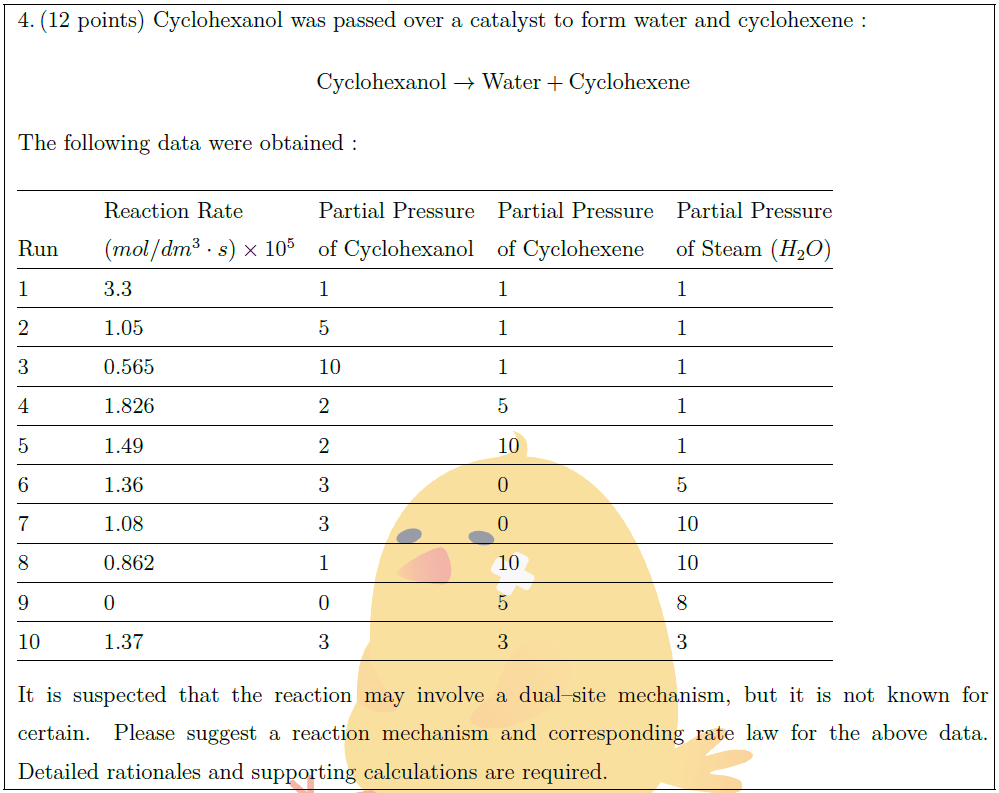
Cyclohexanol was passed over a catalyst to form water and cyclohexene :
\begin{align*}
\mbox{Cyclohexanol} \to \mbox{Water} + \mbox{Cyclohexene}
\end{align*}
The following data were obtained :\\
\begin{tabular}{@{}llllll@{}}
\hline
& & Reaction Rate & Partial Pressure & Partial Pressure & Partial Pressure\\
Run & & $(mol/dm^3 \cdot s) \times 10^5$ & of Cyclohexanol & of Cyclohexene & of Steam ($H_2O$)\\
\hline
1 & & 3.3 & 1 & 1 & 1 \\
\hline
2 & & 1.05 & 5 & 1 & 1 \\
\hline
3 & & 0.565 & 10 & 1 & 1 \\
\hline
4 & & 1.826 & 2 & 5 & 1 \\
\hline
5 & & 1.49 & 2 & 10 & 1 \\
\hline
6 & & 1.36 & 3 & 0 & 5 \\
\hline
7 & & 1.08 & 3 & 0 & 10 \\
\hline
8 & & 0.862 & 1 & 10 & 10 \\
\hline
9 & & 0 & 0 & 5 & 8 \\
\hline
10 & & 1.37 & 3 & 3 & 3\\
\hline
\end{tabular}\\
\\
It is suspected that the reaction may involve a dual–site mechanism, but it is not known for certain. Please suggest a reaction mechanism and corresponding rate law for the above data. Detailed rationales and supporting calculations are required.