Solution:
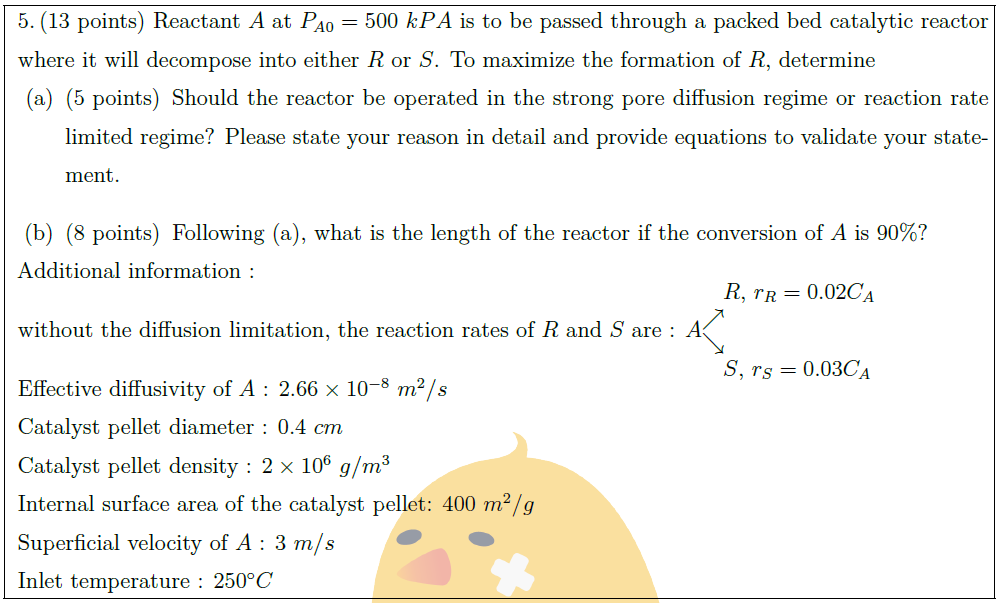
Reactant $A$ at $P_{A0} = 500\ k PA$ is to be passed through a packed bed catalytic reactor where it will decompose into either $R$ or $S$. To maximize the formation of $R$, determine
\begin{parts}
\part [5] Should the reactor be operated in the strong pore diffusion regime or reaction rate limited regime? Please state your reason in detail and provide equations to validate your statement.
\part [8] Following (a), what is the length of the reactor if the conversion of $A$ is $90 \%$?
\end{parts}
Additional information :\vspace*{-0.8em}\\
\hspace*{31em}$R$, $r_R = 0.02 C_A$\\
without the diffusion limitation, the reaction rates of $R$ and $S$ are : $A^{\displaystyle \nearrow}_{\displaystyle \searrow}$\\
\hspace*{31em}$S$, $r_S = 0.03 C_A$\vspace*{-0.8em}\\
Effective diffusivity of $A$ : $2.66 \times 10^{-8}\ m^2/s$\\
Catalyst pellet diameter : $0.4\ cm$\\
Catalyst pellet density : $2 \times 10^6\ g/m^3$\\
Internal surface area of the catalyst pellet: $400\ m^2/g$\\
Superficial velocity of $A$ : $3\ m/s$\\
Inlet temperature : $250^\circ C$