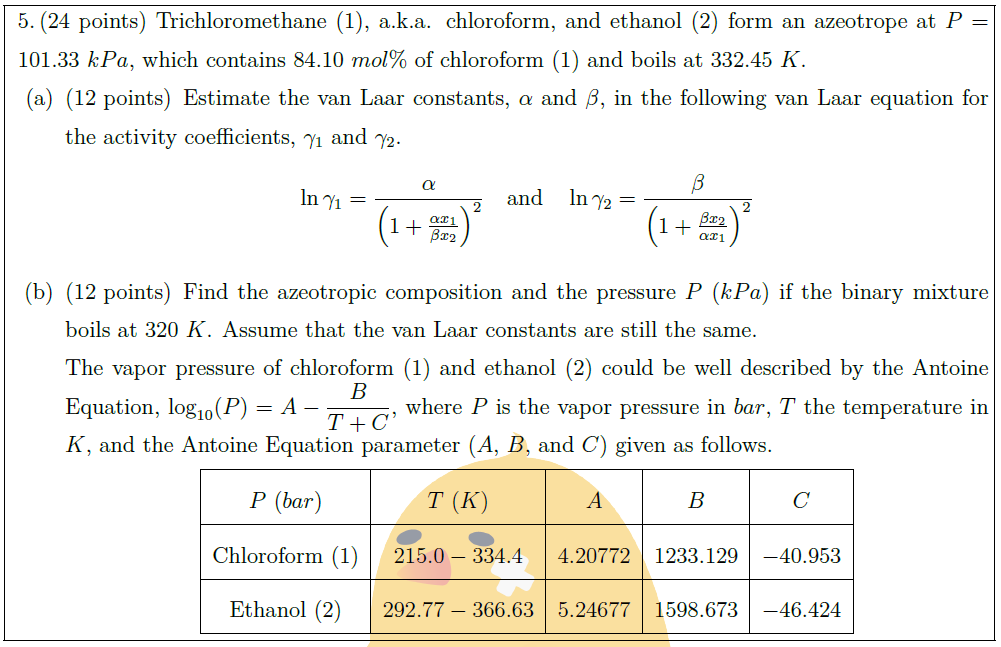
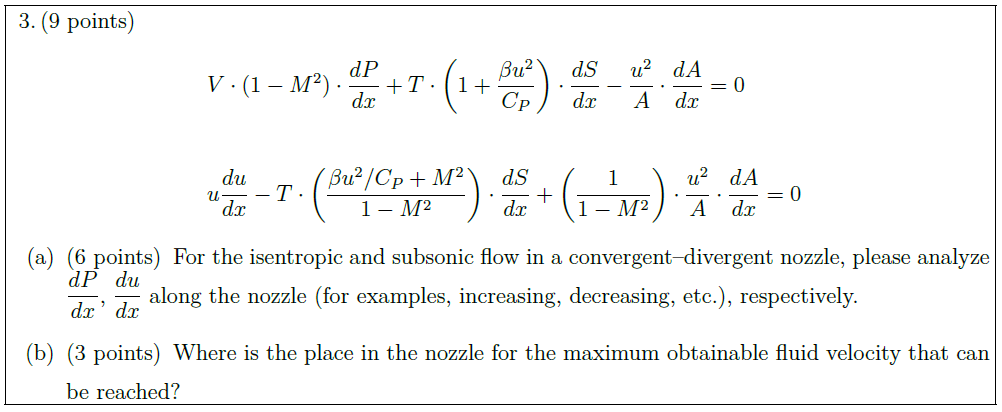
Solution:
In a binary mixture, $P_A$ and $P_B$ are the partial vapor pressure of the two constituents, and $x_A$ and $x_B$ are the mole fractions of the liquid. Assume that the vapor mixture could be regarded as an ideal gas.\vspace*{0.5em}\\
Please express $\displaystyle \left( \frac{d \ln P_A}{d \ln P_B} \right)_{T,P}$ as a function of $x_A$ and $x_B$.