Solution:
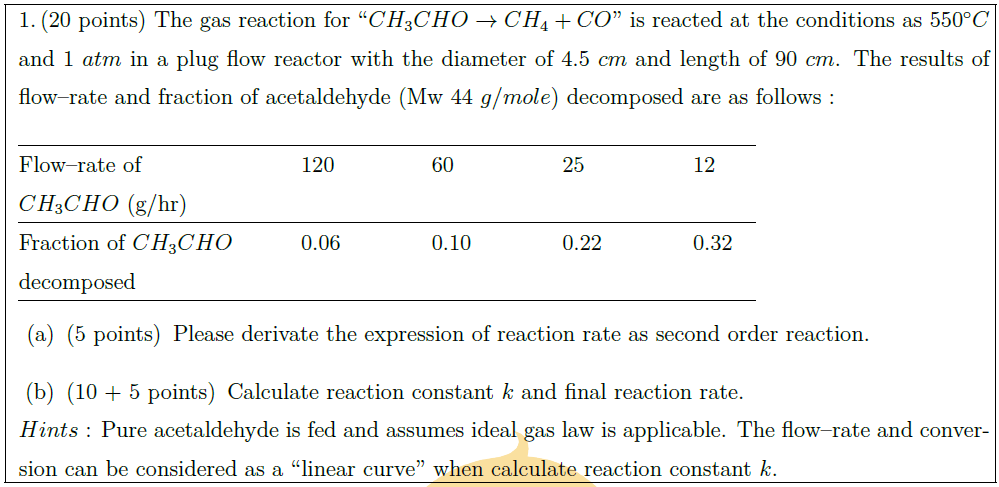
The gas reaction for “$CH_3CHO \to CH_4 + CO$” is reacted at the conditions as $550^\circ C$ and $1\ atm$ in a plug flow reactor with the diameter of $4.5\ cm$ and length of $90\ cm$. The results of flow–rate and fraction of acetaldehyde (Mw $44\ g/mole$) decomposed are as follows :\\
\begin{tabular}{@{}lllll@{}}
\hline
Flow–rate of & \quad\quad 120\quad\quad & \quad\quad 60\quad\quad & \quad\quad 25\quad\quad & \quad\quad 12\quad\quad \\
$CH_3CHO$ (g/hr) & & & & \\
\hline
Fraction of $CH_3CHO$ & \quad\quad 0.06\quad\quad & \quad\quad 0.10\quad\quad & \quad\quad 0.22\quad\quad & \quad\quad 0.32\quad\quad \\
decomposed & & & &\\
\hline
\end{tabular}\\
\begin{parts}
\part[5] Please derivate the expression of reaction rate as second order reaction.
\part[10 + 5] Calculate reaction constant $k$ and final reaction rate.
\end{parts}
$Hints$ : Pure acetaldehyde is fed and assumes ideal gas law is applicable. The flow–rate and conversion can be considered as a “linear curve” when calculate reaction constant $k$.