Solution:
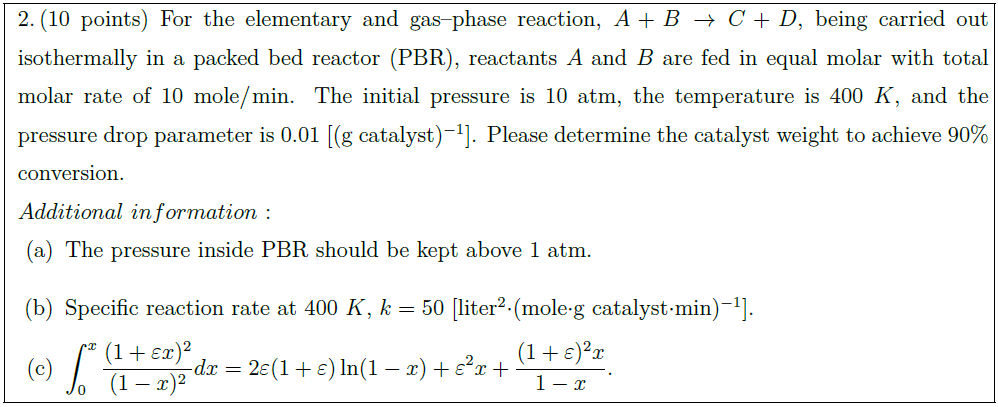
For the elementary and gas–phase reaction, $A + B \to C + D$, being carried out isothermally in a packed bed reactor (PBR), reactants $A$ and $B$ are fed in equal molar with total molar rate of 10 mole/min. The initial pressure is 10 atm, the temperature is $400\ K$, and the pressure drop parameter is 0.01 [(g catalyst)$^{-1}$]. Please determine the catalyst weight to achieve 90$\%$ conversion.\\
$Additional\ information$ :
\begin{parts}
\part The pressure inside PBR should be kept above 1 atm.
\part Specific reaction rate at $400\ K$, $k = 50$ [liter$^2 \cdot$(mole$\cdot$g catalyst$\cdot$min)$^{-1}$].
\part $\displaystyle \int_0^x \frac{(1 + \varepsilon x)^2}{(1 – x)^2} dx = 2 \varepsilon (1 + \varepsilon) \ln (1 – x) + \varepsilon^2 x + \frac{(1 + \varepsilon)^2 x}{1 – x}$.
\end{parts}