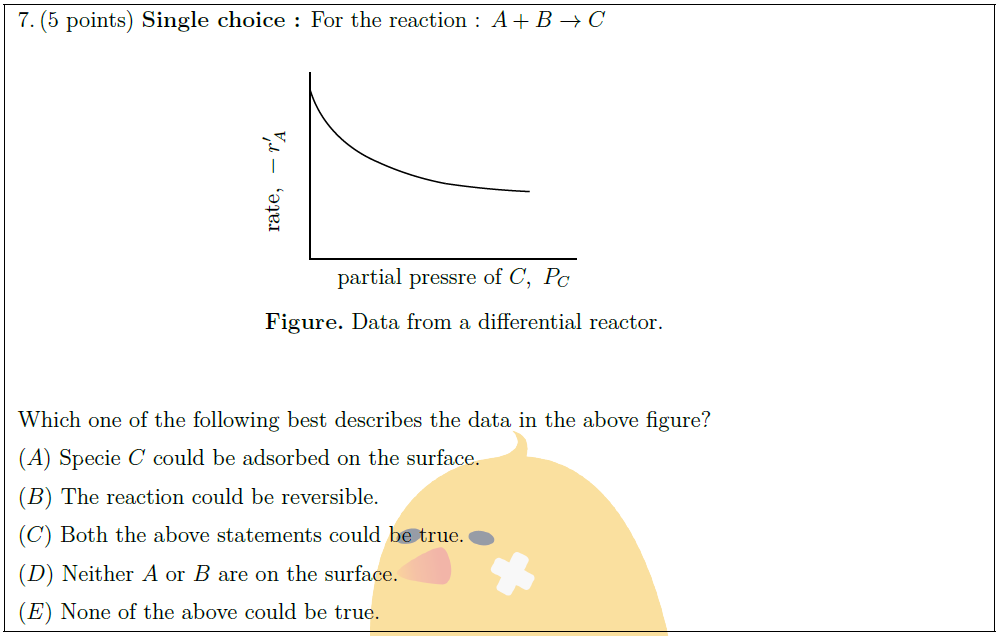
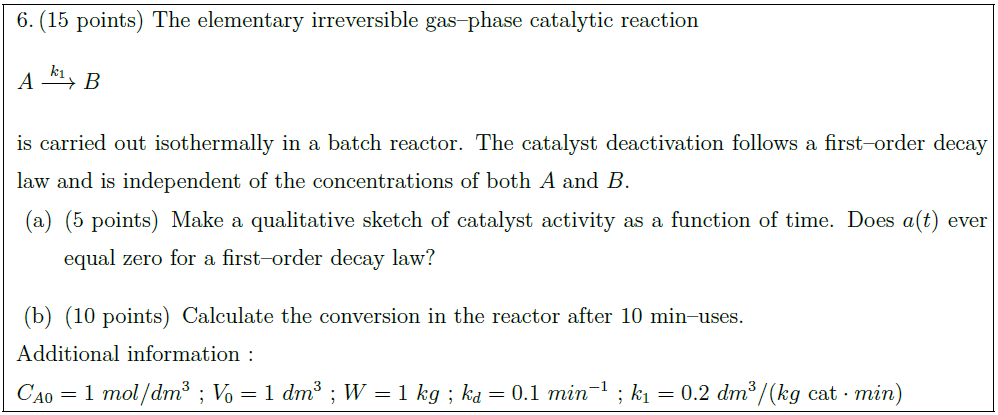
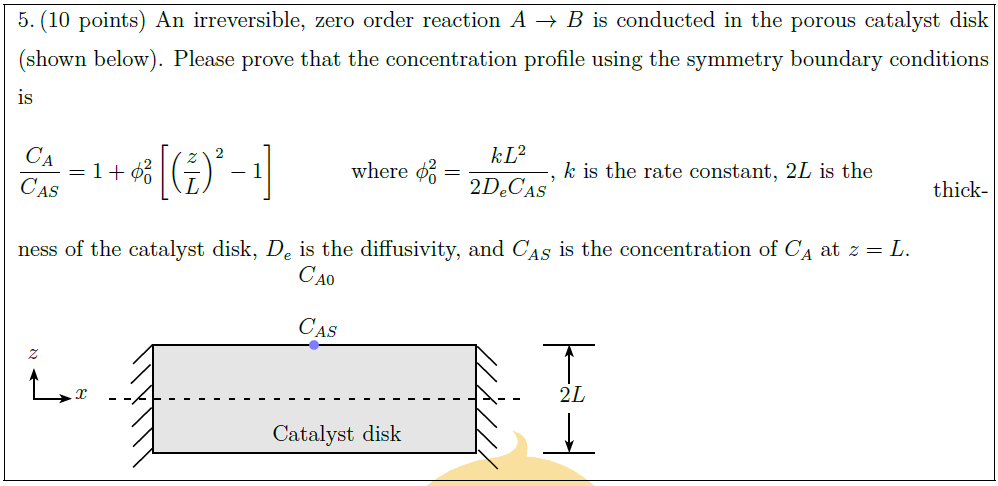
Solution:
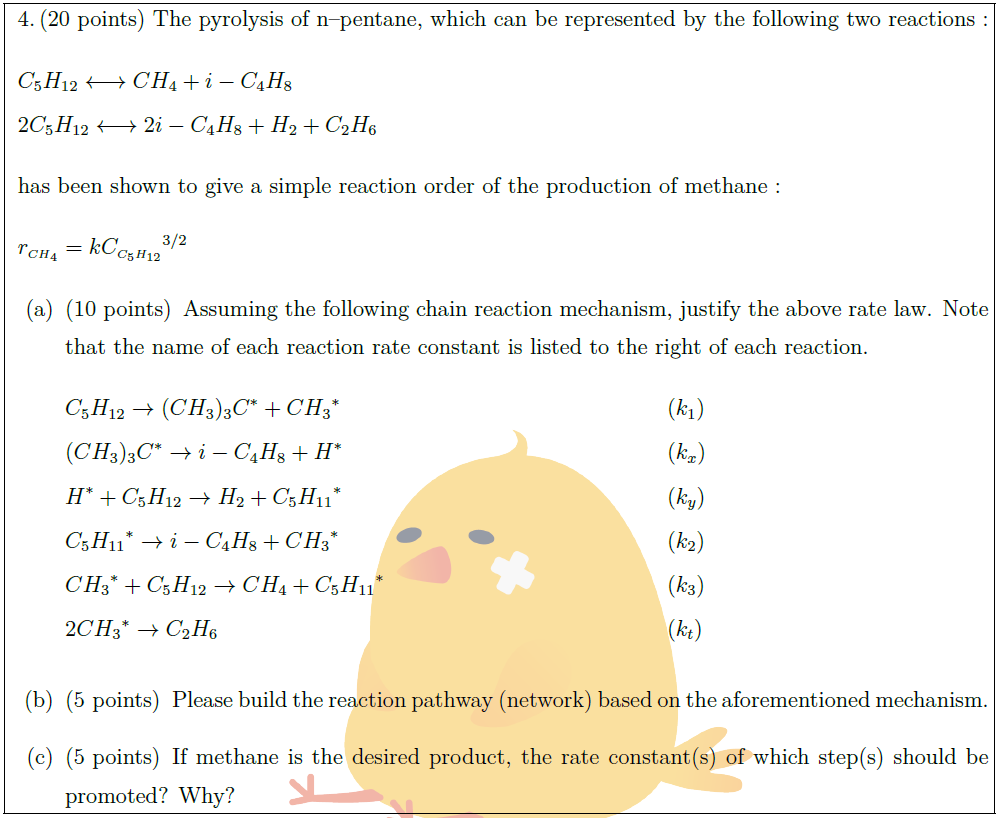
The pyrolysis of n–pentane, which can be represented by the following two reactions :
\begin{flalign*}
& C_5H_{12} \longleftrightarrow CH_4 + i-C_4H_8 &\\
& 2C_5H_{12} \longleftrightarrow 2 i-C_4H_8 + H_2 + C_2H_6 &
\end{flalign*}
has been shown to give a simple reaction order of the production of methane :
\begin{flalign*}
& r_{\scriptscriptstyle CH_4} = k {C_{\scriptscriptstyle C_5H_{12}}}^{3/2} &
\end{flalign*}
\begin{parts}
\part [10] Assuming the following chain reaction mechanism, justify the above rate law. Note that the name of each reaction rate constant is listed to the right of each reaction.
\begin{flalign*}
& C_5H_{12} \to (CH_3)_3C^{*} + {CH_3}^{*} & & (k_1) &\\
& (CH_3)_3C^{*} \to i-C_4H_8 + H^{*} & & (k_x) &\\
& H^{*} + C_5H_{12} \to H_2 + {C_5H_{11}}^{*} & & (k_y) &\\
& {C_5H_{11}}^{*} \to i-C_4H_8 + {CH_3}^{*} & & (k_2) &\\
& {CH_3}^{*} + C_5H_{12} \to CH_4 + {C_5H_{11}}^{*} & & (k_3) &\\
& 2{CH_3}^{*} \to C_2H_6 & & (k_t) &
\end{flalign*}
\part [5] Please build the reaction pathway (network) based on the aforementioned mechanism.
\part [5] If methane is the desired product, the rate constant(s) of which step(s) should be promoted? Why?
\end{parts}