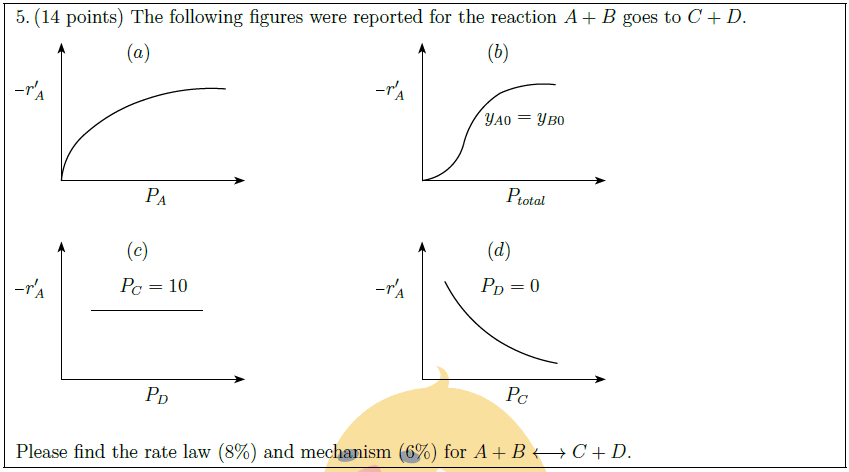
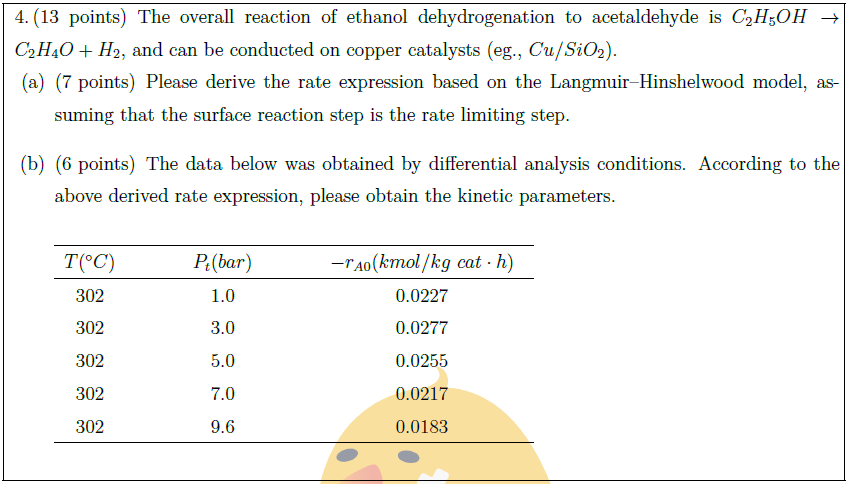
Solution:
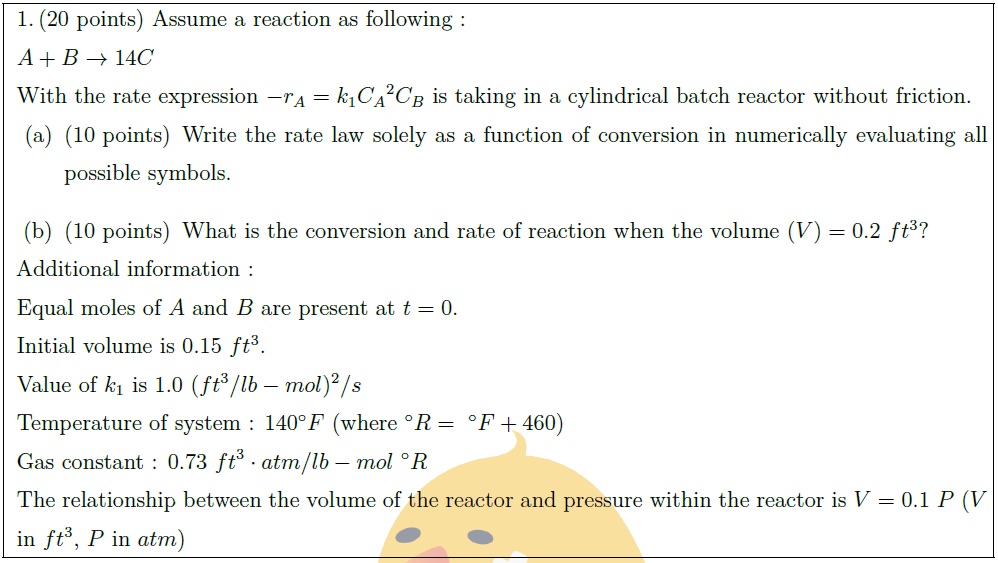
Assume a reaction as following :\\
$A + B \to 14C$\\
With the rate expression $- r_A = k_1 {C_A}^2 C_B$ is taking in a cylindrical batch reactor without friction.
\begin{parts}
\part [10] Write the rate law solely as a function of conversion in numerically evaluating all possible symbols.
\part [10] What is the conversion and rate of reaction when the volume $(V) = 0.2\ ft^3$?
\end{parts}
Additional information :\\
Equal moles of $A$ and $B$ are present at $t = 0$.\\
Initial volume is $0.15\ ft^3$.\\
Value of $k_1$ is $1.0\ (ft^3/lb-mol)^2 / s$\\
Temperature of system : $140^\circ F$ (where $^\circ R =\ ^\circ F + 460$)\\
Gas constant : $0.73\ {ft}^3 \cdot atm / lb-mol\ ^\circ R$\\
The relationship between the volume of the reactor and pressure within the reactor is $V = 0.1\ P$ ($V$ in $ft^3$, $P$ in $atm$)