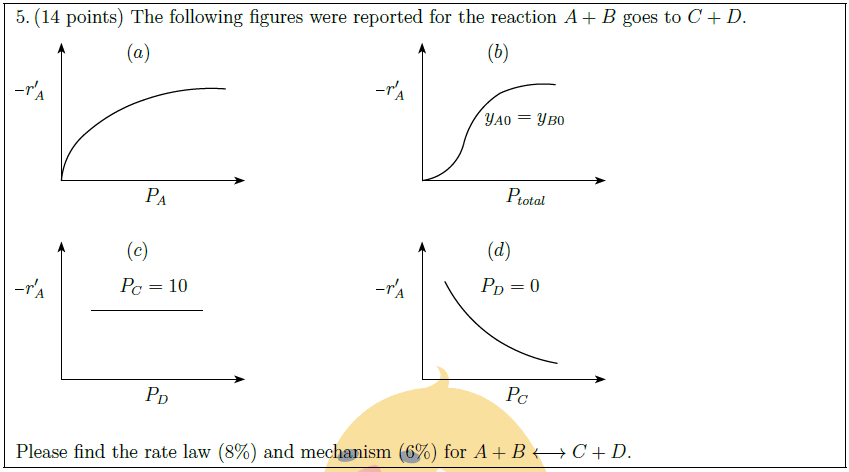
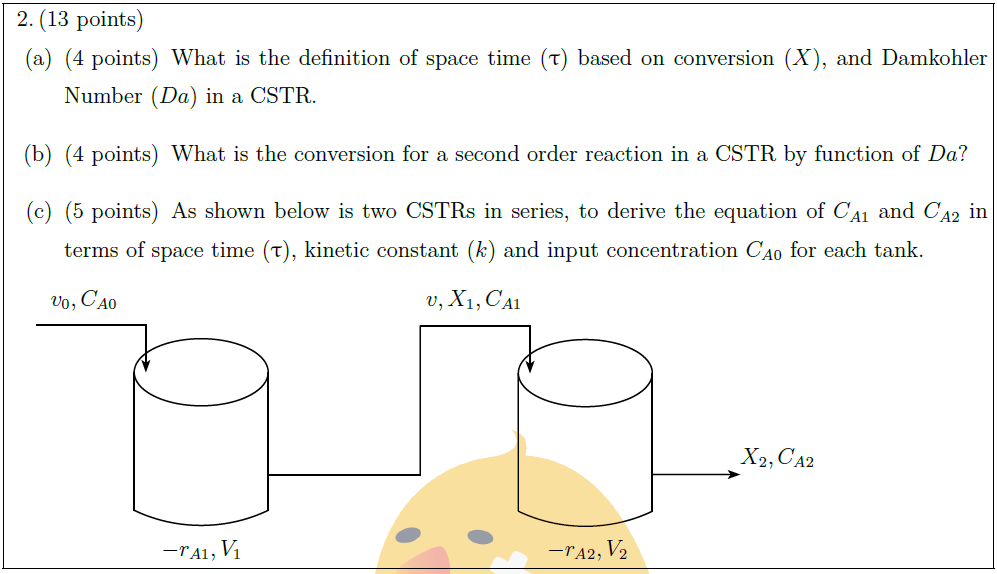
Solution:
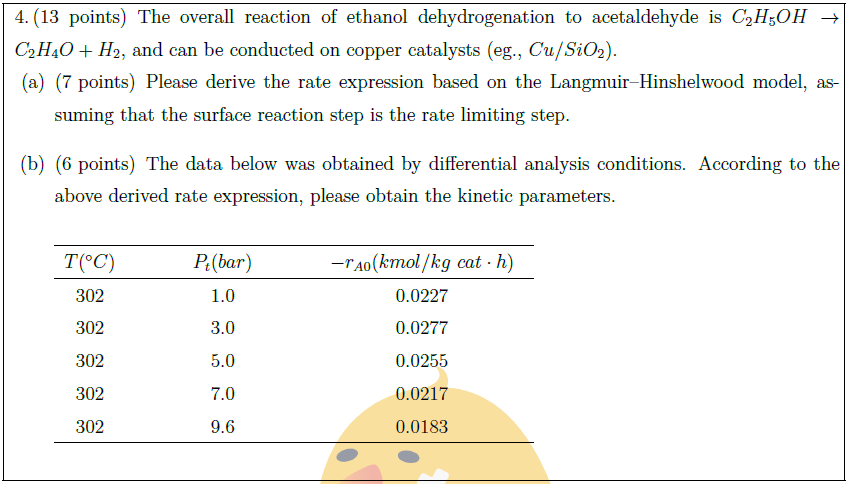
The overall reaction of ethanol dehydrogenation to acetaldehyde is $C_2H_5OH \to C_2H_4O + H_2$, and can be conducted on copper catalysts (eg., $Cu/SiO_2$).
\begin{parts}
\part [7] Please derive the rate expression based on the Langmuir–Hinshelwood model, assuming that the surface reaction step is the rate limiting step.
\part [6] The data below was obtained by differential analysis conditions. According to the above derived rate expression, please obtain the kinetic parameters.\\
\begin{tabular}{cccccccccc@{}}
\hline
$T (^\circ C)$ & & & & $P_t (bar)$ & & & & $- r_{A0} (kmol/kg\ cat \cdot h)$ & \\
\hline
302 & & & & 1.0 & & & & 0.0227 & \\
302 & & & & 3.0 & & & & 0.0277 & \\
302 & & & & 5.0 & & & & 0.0255 & \\
302 & & & & 7.0 & & & & 0.0217 & \\
302 & & & & 9.6 & & & & 0.0183 & \\
\hline
\\
\end{tabular}
\end{parts}