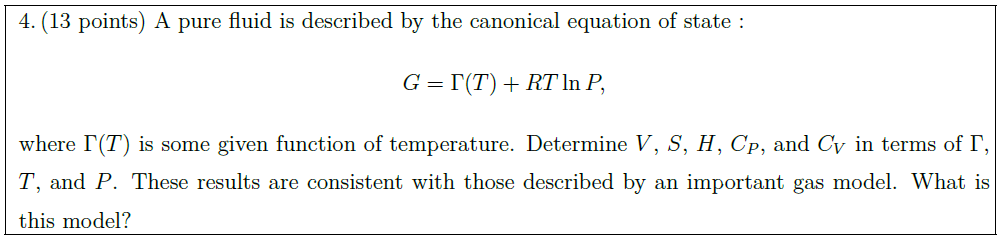
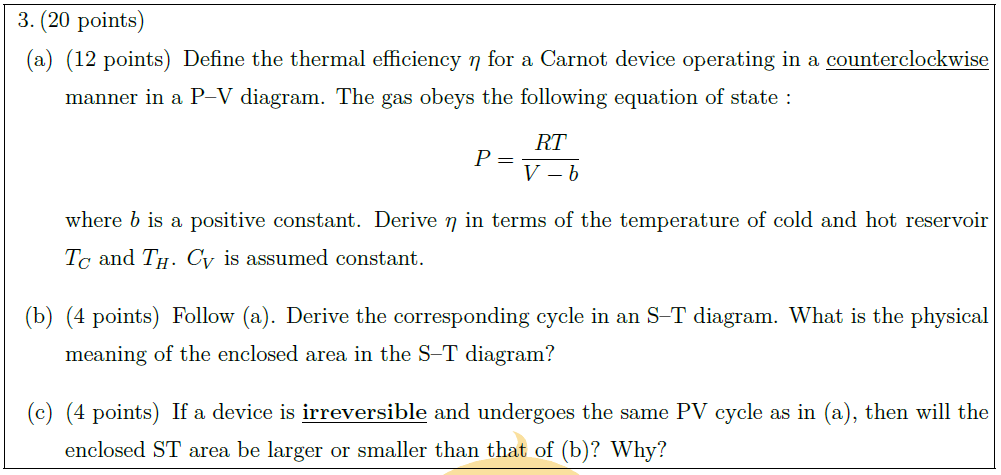
Solution:
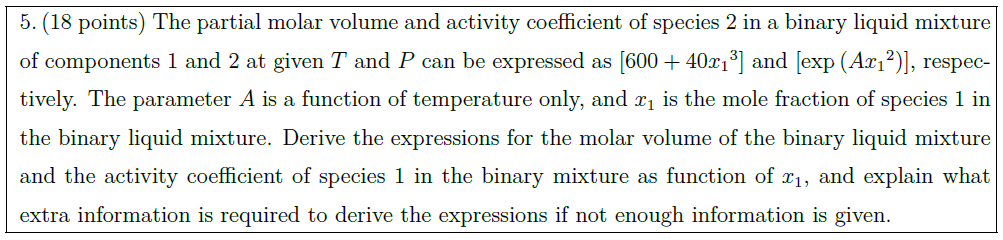
The partial molar volume and activity coefficient of species 2 in a binary liquid mixture of components 1 and 2 at given $T$ and $P$ can be expressed as $\left[ 600 + 40 {x_1}^3 \right]$ and $\left[ \exp \left( A {x_1}^2 \right) \right]$, respectively. The parameter $A$ is a function of temperature only, and $x_1$ is the mole fraction of species 1 in the binary liquid mixture. Derive the expressions for the molar volume of the binary liquid mixture and the activity coefficient of species 1 in the binary mixture as function of $x_1$, and explain what extra information is required to derive the expressions if not enough information is given.