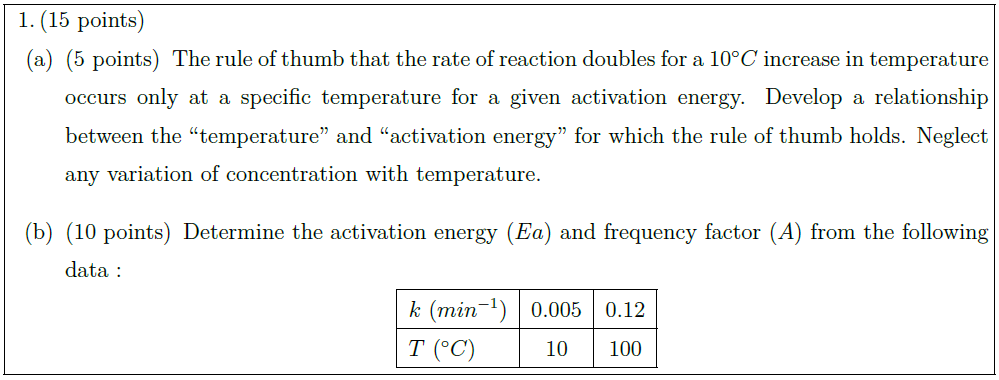
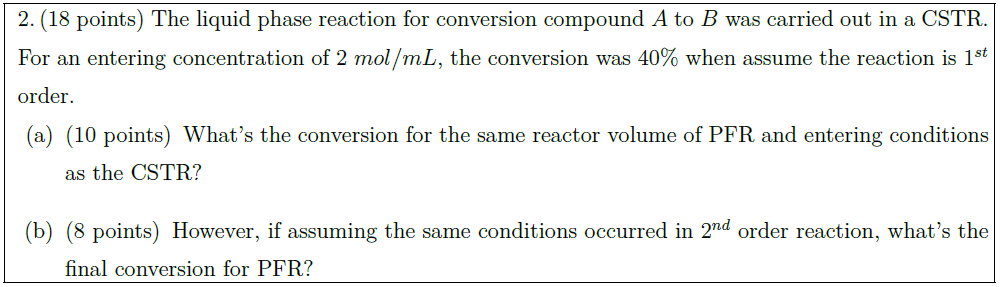
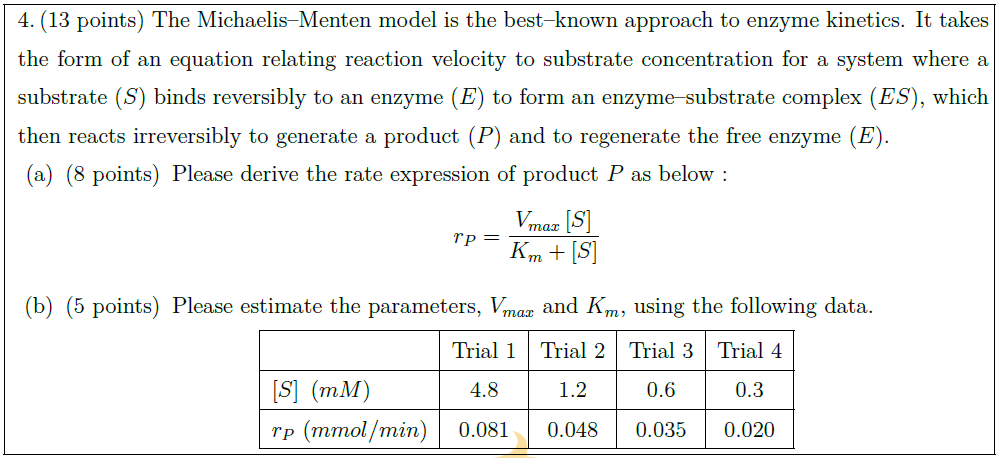
Solution:
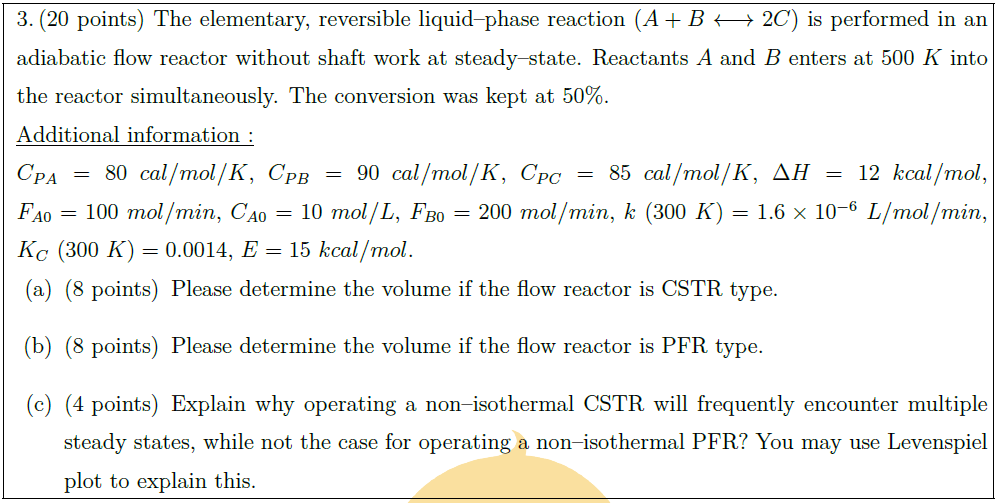
The elementary, reversible liquid–phase reaction ($A + B \longleftrightarrow 2C$) is performed in an adiabatic flow reactor without shaft work at steady–state. Reactants $A$ and $B$ enters at $500\ K$ into the reactor simultaneously. The conversion was kept at $50 \%$.\\
$\uline{\mbox{Additional information :}}$\\
$C_{PA} = 80\ cal/mol/K$, $C_{PB} = 90\ cal/mol/K$, $C_{PC} = 85\ cal/mol/K$, $\Delta H = 12\ kcal/mol$, $F_{A0} = 100\ mol/min$, $C_{A0} = 10\ mol/L$, $F_{B0} = 200\ mol/min$, $k\ (300\ K) = 1.6 \times 10^{-6}\ L/mol/min$, $K_C\ (300\ K) = 0.0014$, $E = 15\ kcal/mol$.
\begin{parts}
\part [8] Please determine the volume if the flow reactor is CSTR type.
\part [8] Please determine the volume if the flow reactor is PFR type.
\part [4] Explain why operating a non–isothermal CSTR will frequently encounter multiple steady states, while not the case for operating a non–isothermal PFR? You may use Levenspiel plot to explain this.
\end{parts}