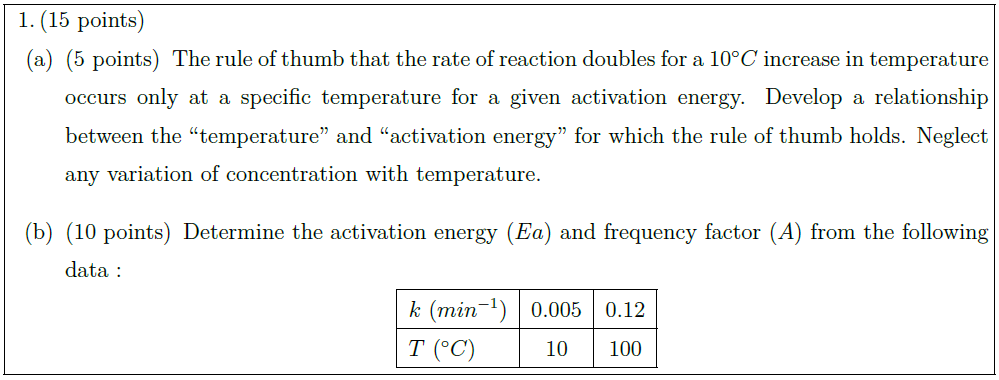
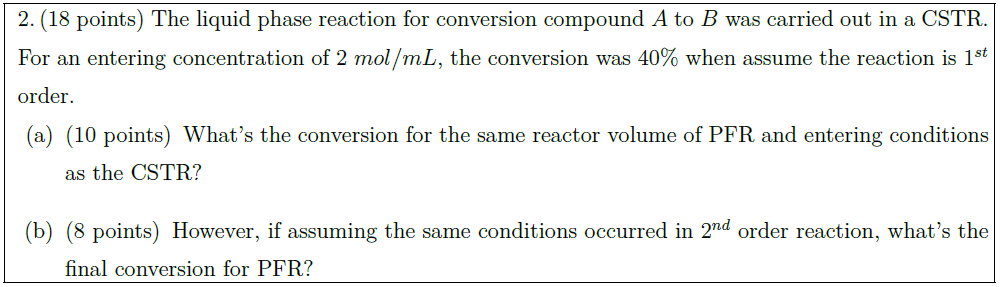
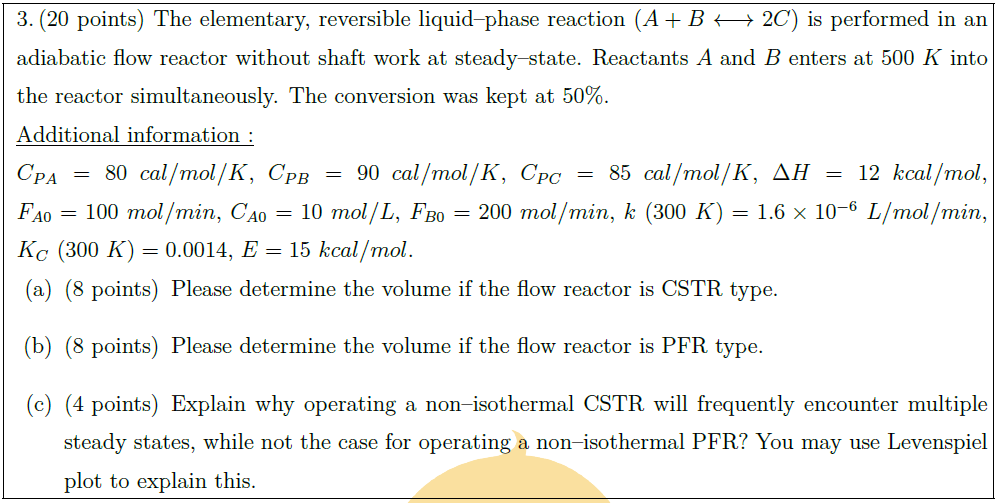
Solution:
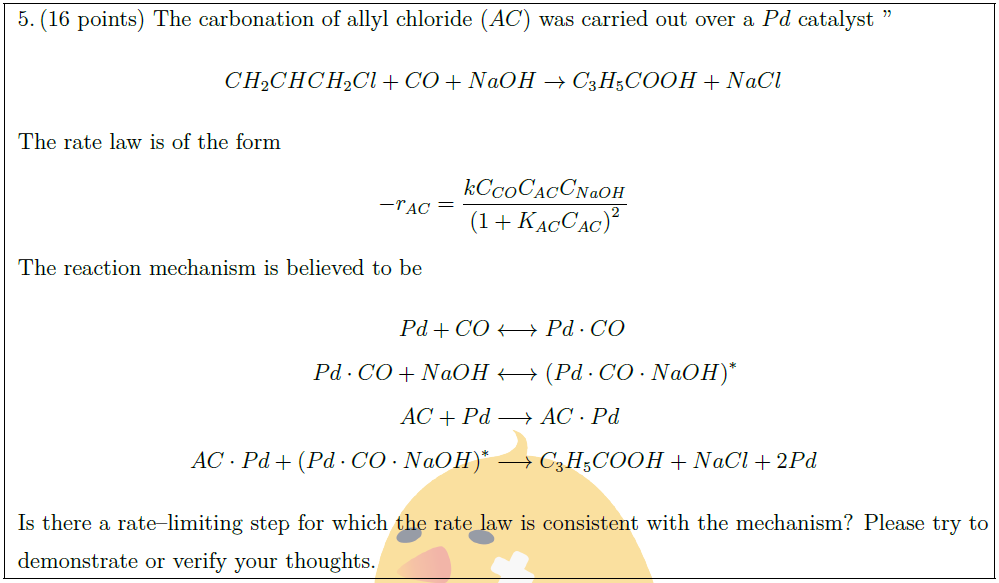
The carbonation of allyl chloride ($AC$) was carried out over a $Pd$ catalyst “
\begin{align*}
CH_2CHCH_2Cl + CO + NaOH \to C_3H_5COOH + NaCl
\end{align*}
The rate law is of the form
\begin{align*}
– r_{AC} = \frac{k C_{CO} C_{AC} C_{NaOH}}{\left( 1 + K_{AC} C_{AC} \right)^2}
\end{align*}
The reaction mechanism is believed to be
\begin{align*}
Pd + CO & \longleftrightarrow Pd \cdot CO\\
Pd \cdot CO + NaOH & \longleftrightarrow \left( Pd \cdot CO \cdot NaOH \right)^*\\
AC + Pd & \longrightarrow AC \cdot Pd\\
AC \cdot Pd + \left( Pd \cdot CO \cdot NaOH \right)^* & \longrightarrow
C_3H_5COOH + NaCl + 2Pd
\end{align*}
Is there a rate–limiting step for which the rate law is consistent with the mechanism? Please try to demonstrate or verify your thoughts.