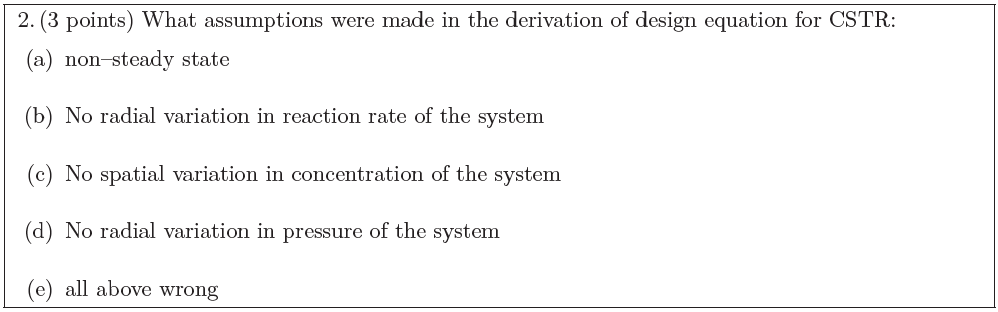
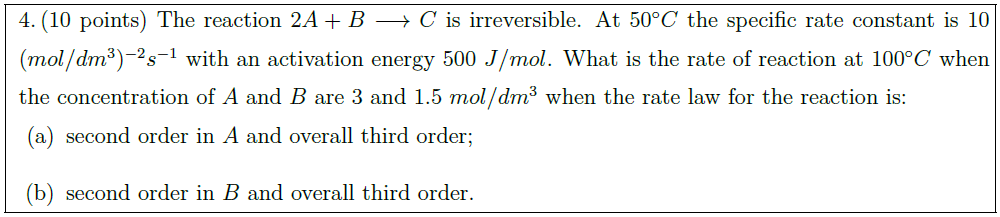
Solution:
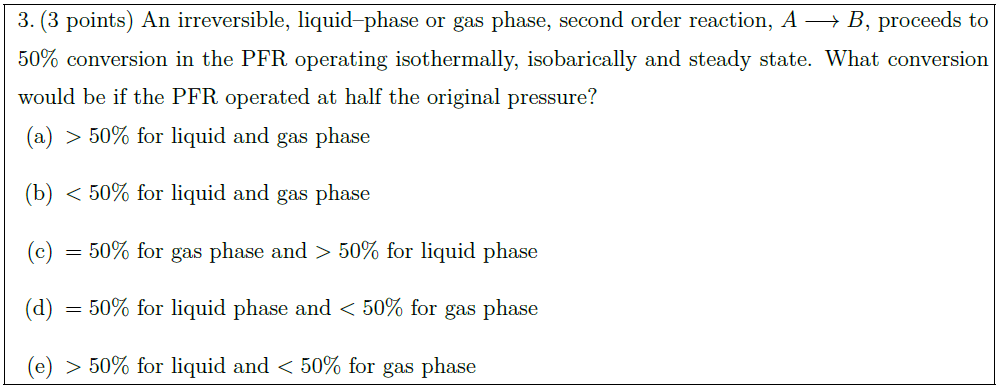
An irreversible, liquid–phase or gas phase, second order reaction, $A \longrightarrow B$, proceeds to $50 \%$
conversion in the PFR operating isothermally, isobarically and steady state. What conversion would be if the PFR operated at half the original pressure?
\begin{parts}
\part $> 50\%$ for liquid and gas phase
\part $< 50\%$ for liquid and gas phase
\part $= 50\%$ for gas phase and $> 50\%$ for liquid phase
\part $= 50\%$ for liquid phase and $< 50\%$ for gas phase
\part $> 50\%$ for liquid and $< 50\%$ for gas phase
\end{parts}