Solution:
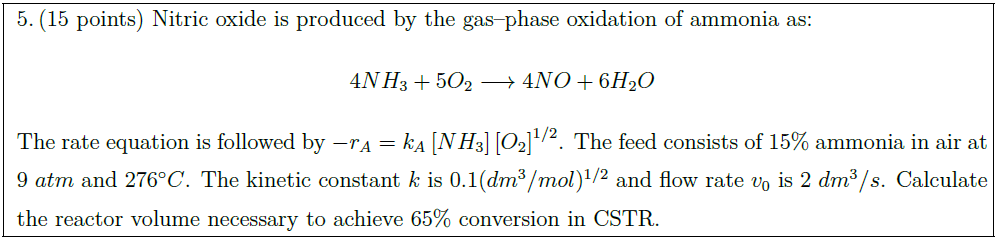
Nitric oxide is produced by the gas–phase oxidation of ammonia as:
\begin{align*}
4 NH_3 + 5 O_2 \longrightarrow 4 NO + 6 H_2O
\end{align*}
The rate equation is followed by $- r_A = k_A \left[ NH_3 \right] \left[ O_2 \right]^{1/2}$. The feed consists of $15 \%$ ammonia in air at $9\ atm$ and $276^\circ C$. The kinetic constant $k$ is $0.1 (dm^3 / mol)^{1/2}$ and flow rate $v_0$ is $2\ dm^3 / s$. Calculate the reactor volume necessary to achieve $65 \%$ conversion in CSTR.