Solution:
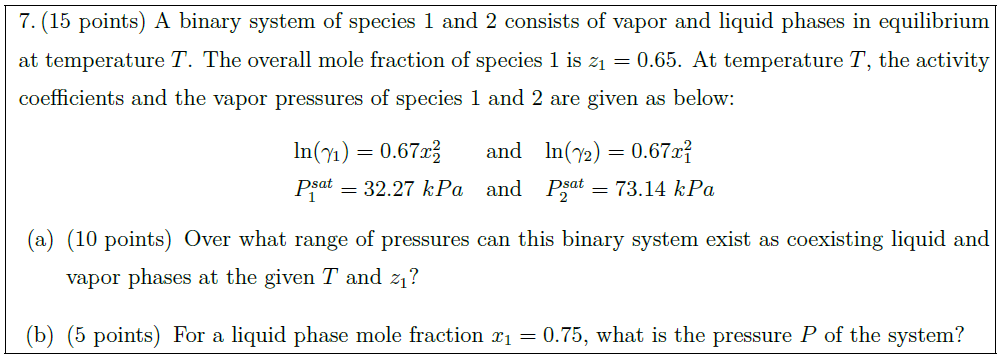
A binary system of species 1 and 2 consists of vapor and liquid phases in equilibrium at temperature $T$. The overall mole fraction of species 1 is $z_1 = 0.65$. At temperature $T$, the activity coefficients and the vapor pressures of species 1 and 2 are given as below:
\begin{center}
\begin{tabular}{@{}lcl@{}}
$\ln (\gamma_1) = 0.67 x_2^2$ & and & $\ln (\gamma_2) = 0.67 x_1^2$\\
$P_1^{sat} = 32.27\ kPa$ & and & $P_2^{sat} = 73.14\ kPa$
\end{tabular}
\end{center}
\begin{parts}
\part [10] Over what range of pressures can this binary system exist as coexisting liquid and vapor phases at the given $T$ and $z_1$?
\part [5] For a liquid phase mole fraction $x_1 = 0.75$, what is the pressure $P$ of the system?
\end{parts}