Solution:
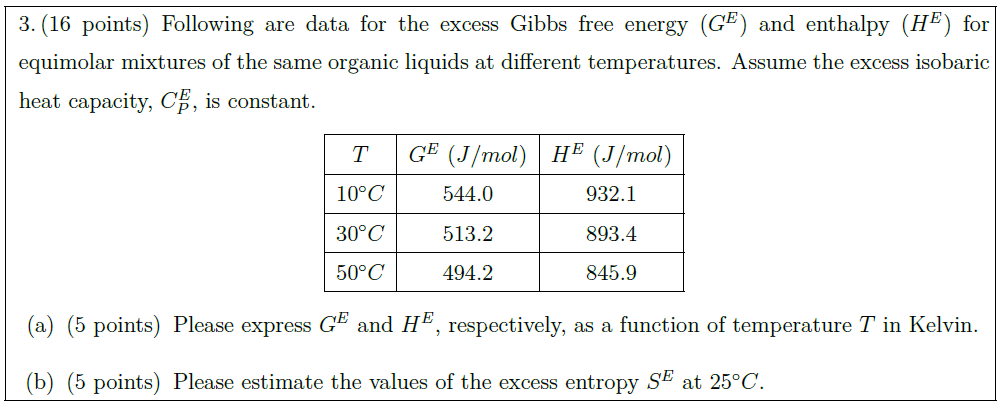
Following are data for the excess Gibbs free energy ($G^E$) and enthalpy ($H^E$) for equimolar mixtures of the same organic liquids at different temperatures. Assume the excess isobaric heat capacity, $C_P^E$, is constant.
\begin{center}
\begin{tabular}{|c|c|c|}
\hline
$T$ & $G^E\ (J / mol)$ & $H^E\ (J /mol)$\\
\hline
$10^\circ C$ & 544.0 & 932.1\\
\hline
$30^\circ C$ & 513.2 & 893.4\\
\hline
$50^\circ C$ & 494.2 & 845.9\\
\hline
\end{tabular}
\end{center}
\begin{parts}
\part [5] Please express $G^E$ and $H^E$, respectively, as a function of temperature $T$ in Kelvin.
\part [5] Please estimate the values of the excess entropy $S^E$ at $25^\circ C$.
\end{parts}