Solution:
\begin{parts}
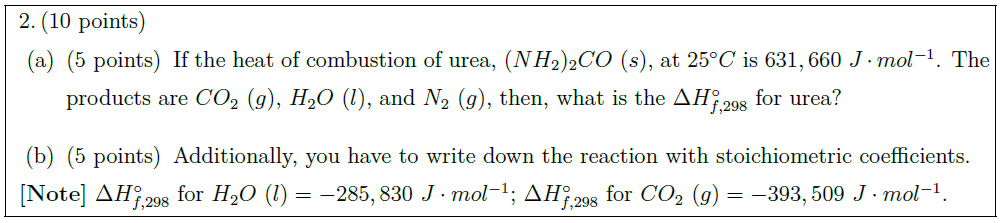
\part [5] If the heat of combustion of urea, $(NH_2)_2 CO\ (s)$, at $25^\circ C$ is $631,660\ J \cdot mol^{-1}$. The products are $CO_2\ (g)$, $H_2O\ (l)$, and $N_2\ (g)$, then, what is the $\Delta H_{f, 298}^\circ$ for urea?
\part [5] Additionally, you have to write down the reaction with stoichiometric coefficients.
\end{parts}
\textbf{[Note]} $\Delta H_{f, 298}^\circ$ for $H_2O\ (l) = – 285,830\ J \cdot mol^{-1}$; $\Delta H_{f, 298}^\circ$ for $CO_2\ (g) = – 393,509\ J \cdot mol^{-1}$.