Solution:
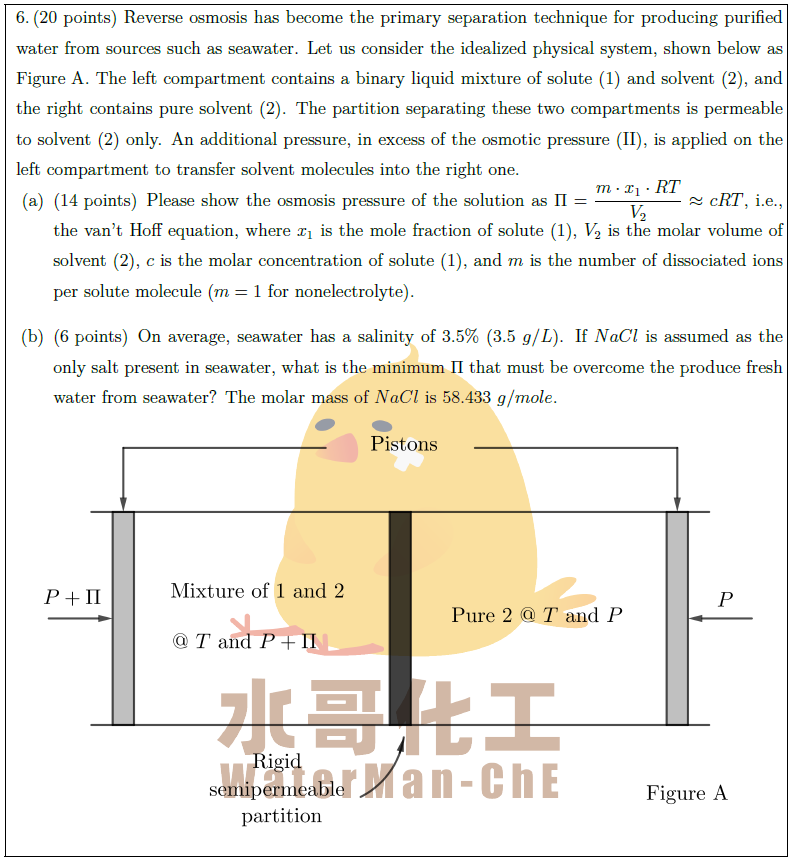
Reverse osmosis has become the primary separation technique for producing purified water from sources such as seawater. Let us consider the idealized physical system, shown below as Figure A. The left compartment contains a binary liquid mixture of solute (1) and solvent (2), and the right contains pure solvent (2). The partition separating these two compartments is permeable to solvent (2) only. An additional pressure, in excess of the osmotic pressure (\uppercase\expandafter{\romannumeral 2}), is applied on the left compartment to transfer solvent molecules into the right one.
\begin{parts}
\part [14] Please show the osmosis pressure of the solution as $\displaystyle \Pi = \frac{m \cdot x_1 \cdot RT}{V_2} \approx cRT$, i.e., the van’t Hoff equation, where $x_1$ is the mole fraction of solute (1), $V_2$ is the molar volume of solvent (2), $c$ is the molar concentration of solute (1), and $m$ is the number of dissociated ions per solute molecule ($m = 1$ for nonelectrolyte).
\part [6] On average, seawater has a salinity of $3.5 \%$ ($3.5\ g / L$). If $NaCl$ is assumed as the only salt present in seawater, what is the minimum $\Pi$ that must be overcome the produce fresh water from seawater? The molar mass of $NaCl$ is $58.433\ g / mole$.