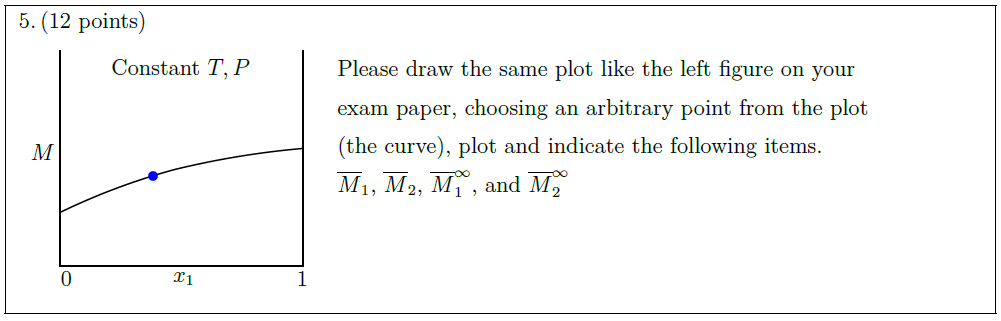
Solution:
The excess enthalpy for a liquid mixture of species 1, 2 at certain fixed $T$ and $P$ is represented by
\begin{align*}
H^E = x_1 x_2 \cdot (20 x_1 + 10 x_2)
\end{align*}
where $H^E$ is in $J \cdot mol^{-1}$. Determine the expression for $\overline{H}_1^E$ and $\overline{H}_2^E$ as functions of $x_1$. Also if
\begin{align*}
H = 10 \cdot (2 x_1 + x_2) \cdot (10 + x_1 x_2)
\end{align*}
Then, please determine the expression for the heat of mixing as function of $x_2$