Solution:
Answer False (F) or True (T). For those “false”, you {\bf{MUST}} give your explanation.
\begin{parts}
\part [4] Turbine needs work. Therefore, the operation of an turbine could cause the increasing of enthalpy from the receiving of work.
\part [4] For an ideal gas, $C_P$ and $C_V$ are constant.
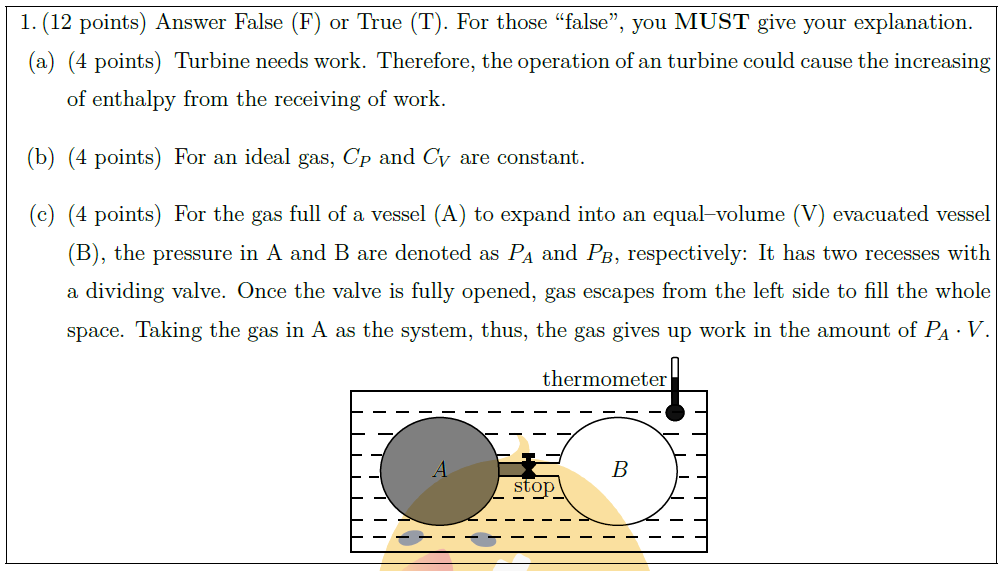
\part [4] For the gas full of a vessel (A) to expand into an equal–volume (V) evacuated vessel (B), the pressure in A and B are denoted as $P_A$ and $P_B$, respectively: It has two recesses with a dividing valve. Once the valve is fully opened, gas escapes from the left side to fill the whole space. Taking the gas in A as the system, thus, the gas gives up work in the amount of $P_A \cdot V$.