Solution:
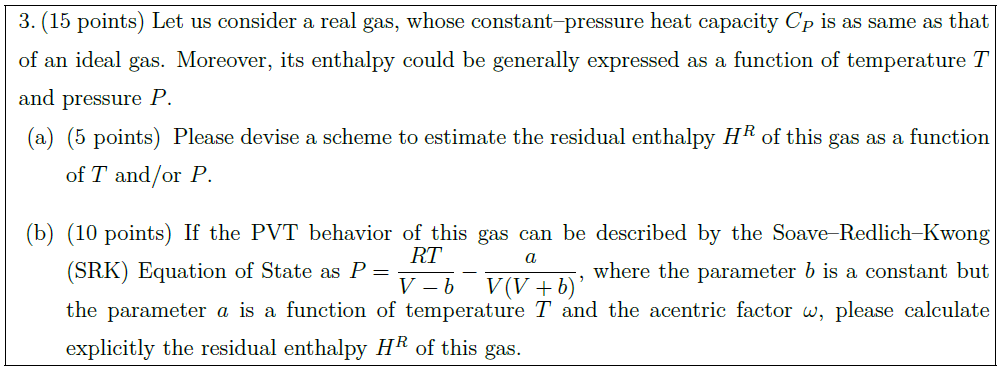
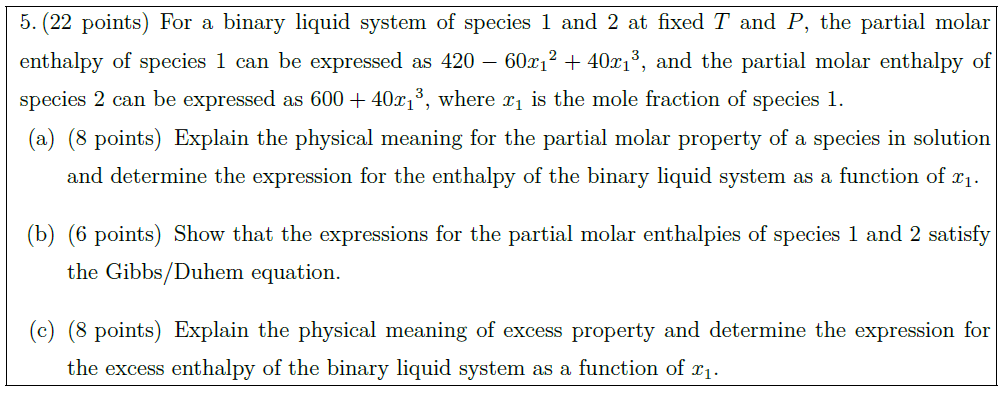
Let us consider a real gas, whose constant–pressure heat capacity $C_P$ is as same as that of an ideal gas. Moreover, its enthalpy could be generally expressed as a function of temperature $T$ and pressure $P$.
\begin{parts}
\part [5] Please devise a scheme to estimate the residual enthalpy $H^R$ of this gas as a function of $T$ and/or $P$.
\part [10] If the PVT behavior of this gas can be described by the Soave–Redlich–Kwong (SRK) Equation of State as $\displaystyle{P = \frac{RT}{V – b} – \frac{a}{V(V + b)}}$, where the parameter $b$ is a constant but the parameter $a$ is a function of temperature $T$ and the acentric factor $\omega$, please calculate explicitly the residual enthalpy $H^R$ of this gas.
\end{parts}