Solution:
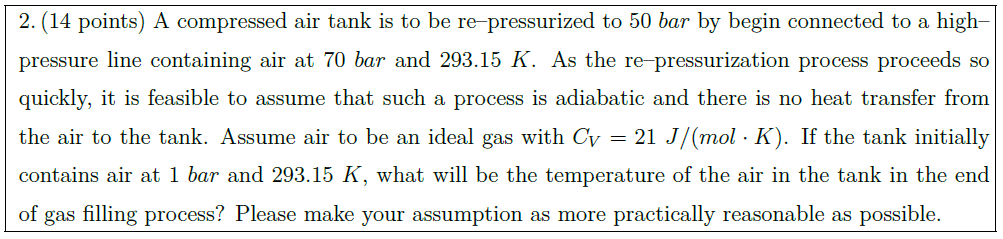
A compressed air tank is to be re–pressurized to $50\ bar$ by begin connected to a high–pressure line containing air at $70\ bar$ and $293.15\ K$. As the re–pressurization process proceeds so quickly, it is feasible to assume that such a process is adiabatic and there is no heat transfer from the air to the tank. Assume air to be an ideal gas with $C_V = 21\ J/(mol \cdot K)$. If the tank initially contains air at $1\ bar$ and $293.15\ K$, what will be the temperature of the air in the tank in the end of gas filling process? Please make your assumption as more practically reasonable as possible.