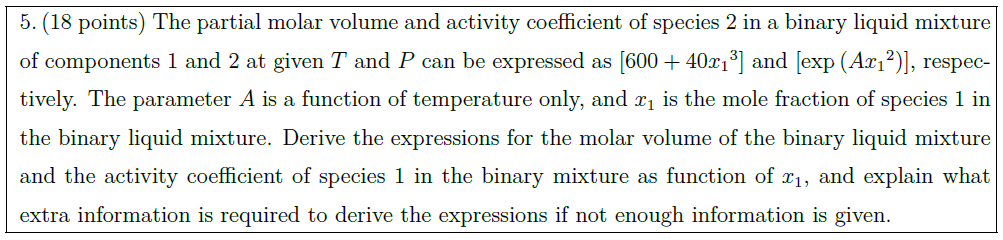
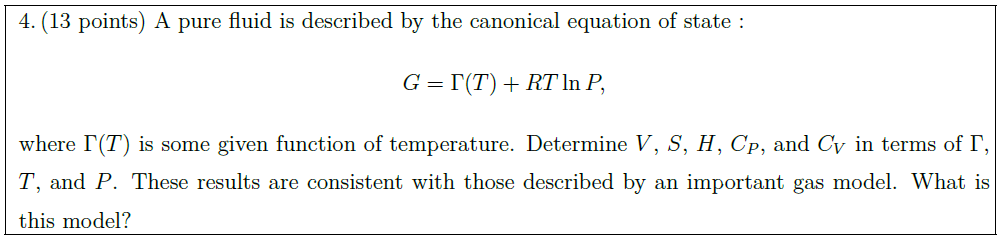
Solution:
\begin{parts}
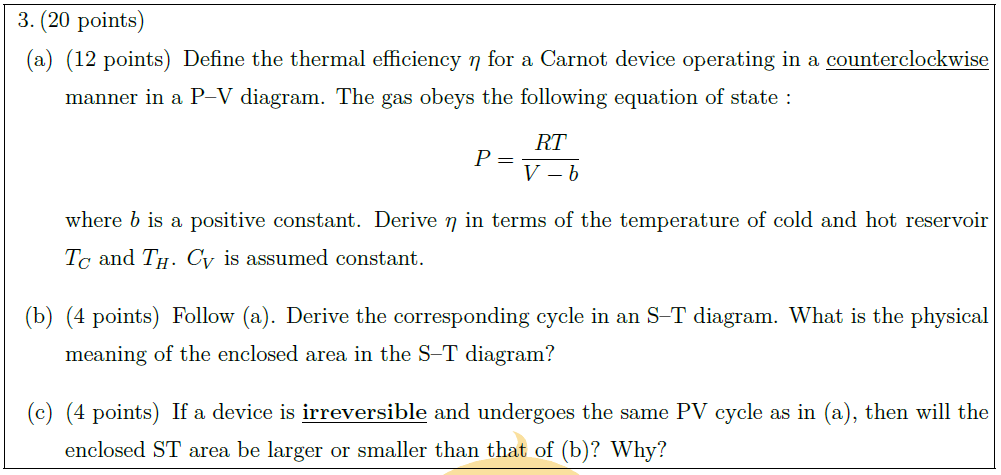
\part [12] Define the thermal efficiency $\eta$ for a Carnot device operating in a $\uline{\mbox{counterclockwise}}$ manner in a P–V diagram. The gas obeys the following equation of state :
$$ P = \frac{RT}{V – b} $$
where $b$ is a positive constant. Derive $\eta$ in terms of the temperature of cold and hot reservoir $T_C$ and $T_H$. $C_V$ is assumed constant.
\part [4] Follow (a). Derive the corresponding cycle in an S–T diagram. What is the physical meaning of the enclosed area in the S–T diagram?
\part [4] If a device is $\uline{\mbox{\textbf{irreversible}}}$ and undergoes the same PV cycle as in (a), then will the enclosed ST area be larger or smaller than that of (b)? Why?
\end{parts}