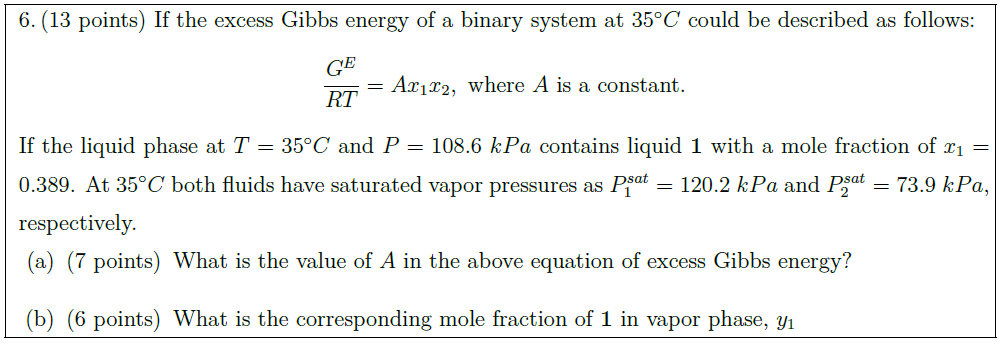
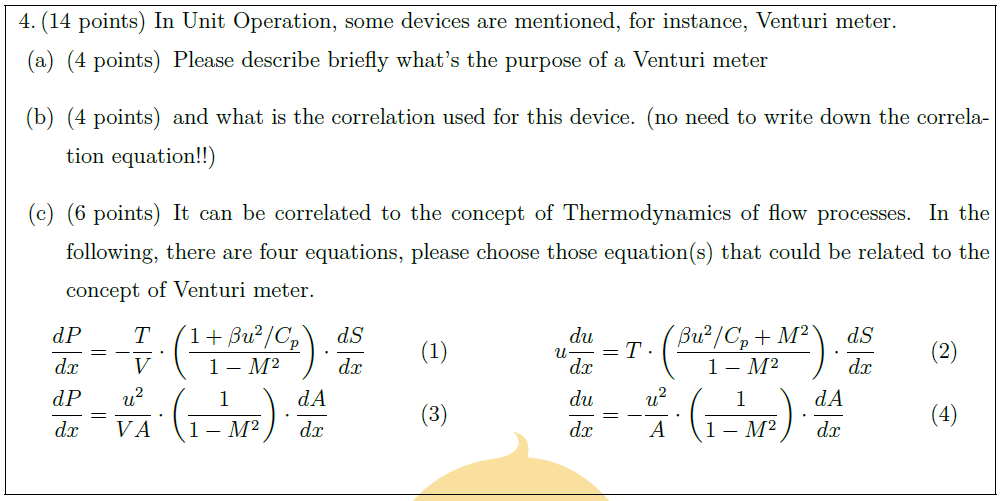
Solution:
One mole of an ideal monatomic gas initially at $300\ K$ and $20\ bar$ is expanded to a final pressure of $1\ bar$. What are the values of $W$, $Q$, $\Delta U$, and $\Delta H$ in the following cases?
\begin{parts}
\part [5] The expansion is reversibly isothermal.
\part [5] The expansion is free isothermally.
\part [7] The gas is expanded adiabatically against an external pressure of $1\ bar$.
\end{parts}