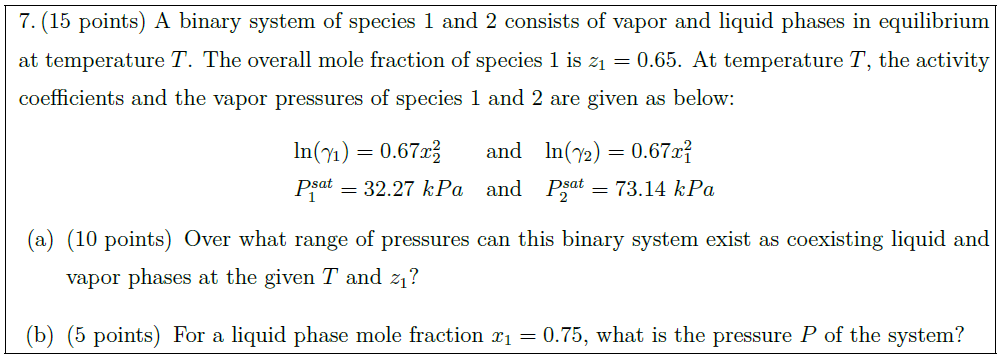
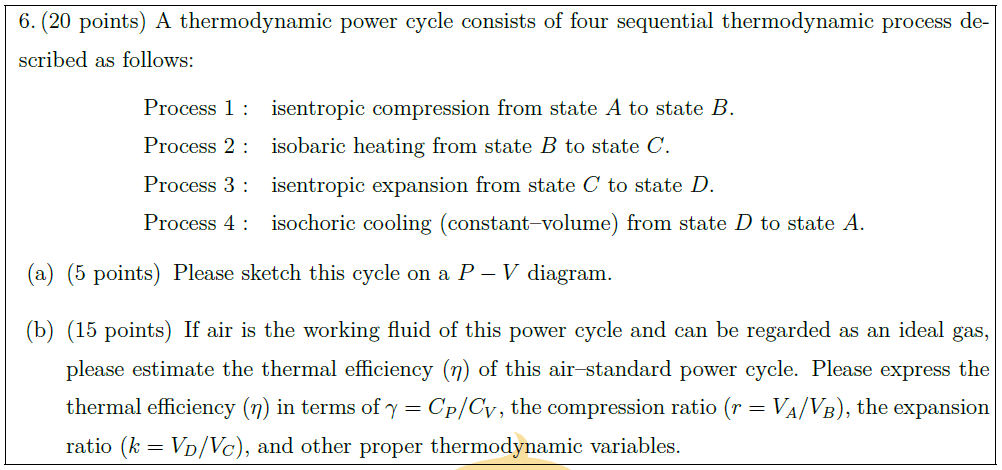
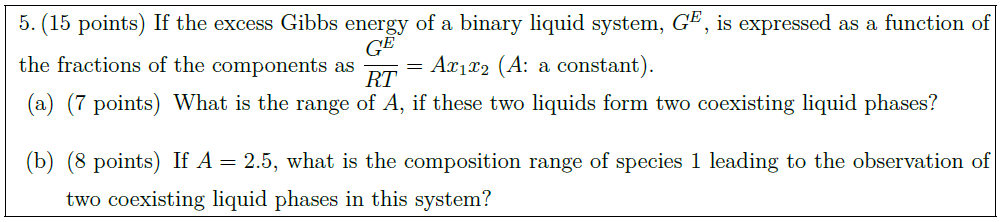
Solution:
Answer false (F) or true (T). For those “false”, you {\bf MUST} justify your answer. If the answer is incorrect, the problem is considered wrong (gain zero score).
\begin{parts}
\part [4] $Q = n \Delta H$ comes merely from the result of a constant–pressure closed system (homogeneous, no chemical reaction, and static (no move) are surely the assumptions as well).
\part [4] A Joule–Thomson process is a type of isenthalpic process where a liquid or a gas is cooled as it passes from a lower pressure state to a higher pressure state.
\end{parts}