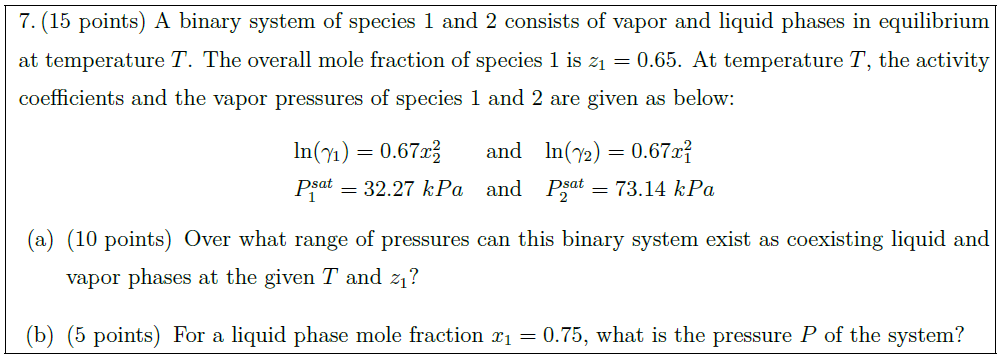
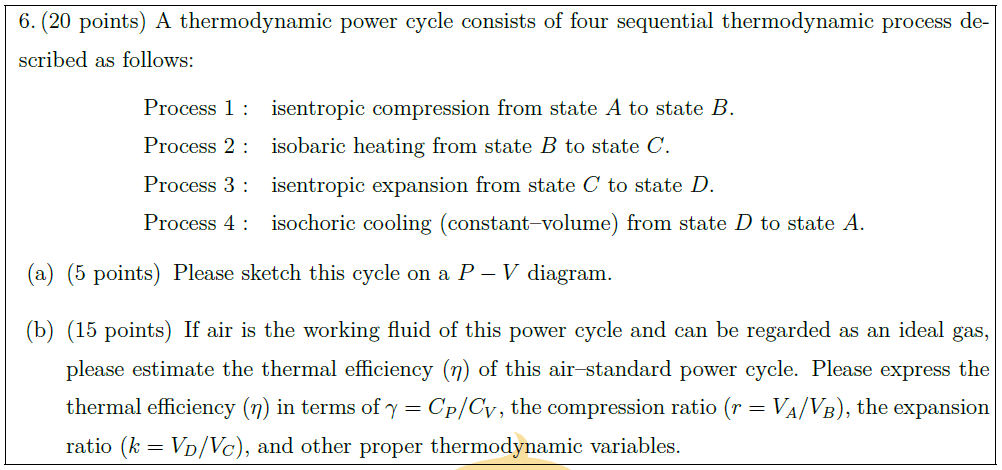
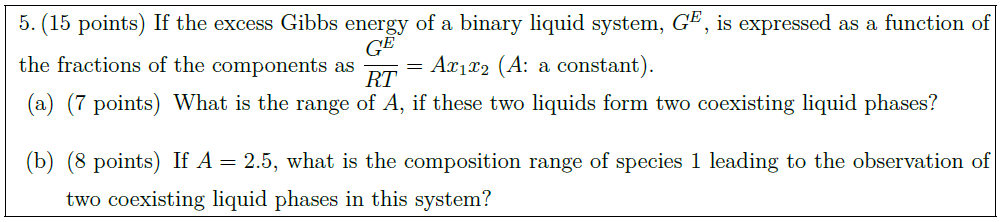
Solution:
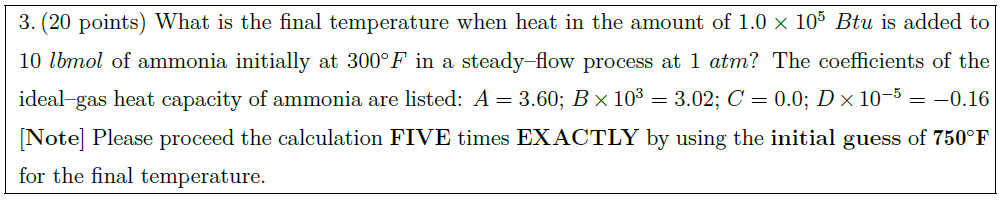
What is the final temperature when heat in the amount of $1.0 \times 10^5\ Btu$ is added to $10\ lbmol$ of ammonia initially at $300^\circ F$ in a steady–flow process at $1\ atm$? The coefficients of the ideal–gas heat capacity of ammonia are listed: $A = 3.60$; $B \times 10^3 = 3.02$; $C = 0.0$; $D \times 10^{-5} = -0.16$\\
$\left[ \mbox{\bf Note} \right]$ Please proceed the calculation {\bf FIVE} times {\bf EXACTLY} by using the {\bf initial guess} of $\mathbf{750^\circ F}$ for the final temperature.