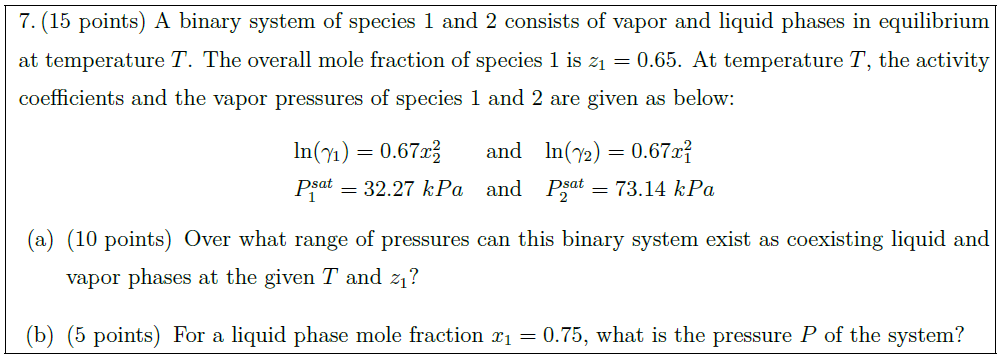
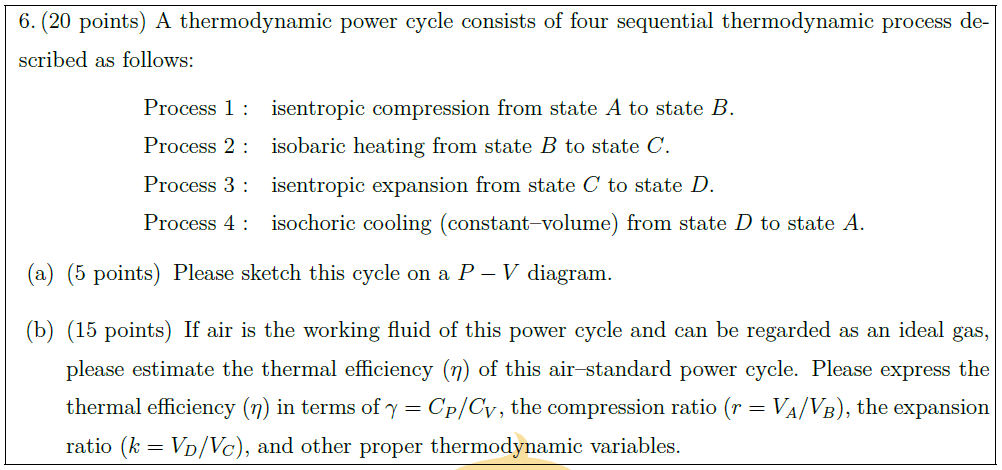
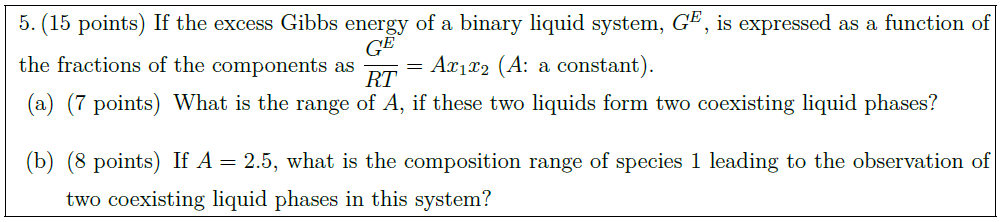
Solution:
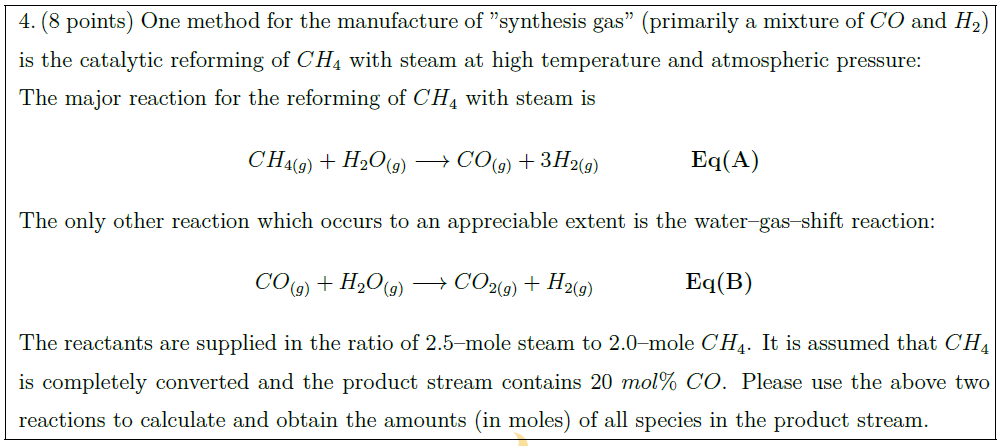
One method for the manufacture of ”synthesis gas” (primarily a mixture of $CO$ and $H_2$) is the catalytic reforming of $CH_4$ with steam at high temperature and atmospheric pressure:\\
The major reaction for the reforming of $CH_4$ with steam is
\begin{align*}
CH_{4 (g)} + H_2O_{(g)} \longrightarrow CO_{(g)} + 3 H_{2 (g)} \quad\quad\quad\quad \mbox{\bf Eq(A)}
\end{align*}
The only other reaction which occurs to an appreciable extent is the water–gas–shift reaction:
\begin{align*}
CO_{(g)} + H_2O_{(g)} \longrightarrow CO_{2 (g)} + H_{2 (g)} \quad\quad\quad\quad \mbox{\bf Eq(B)}
\end{align*}
The reactants are supplied in the ratio of 2.5–mole steam to 2.0–mole $CH_4$. It is assumed that $CH_4$ is completely converted and the product stream contains $20\ mol\%\ CO$. Please use the above two reactions to calculate and obtain the amounts (in moles) of all species in the product stream.