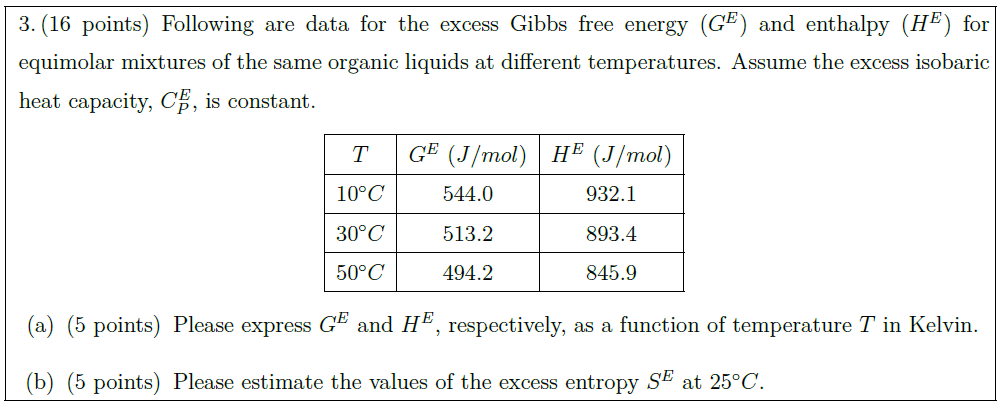
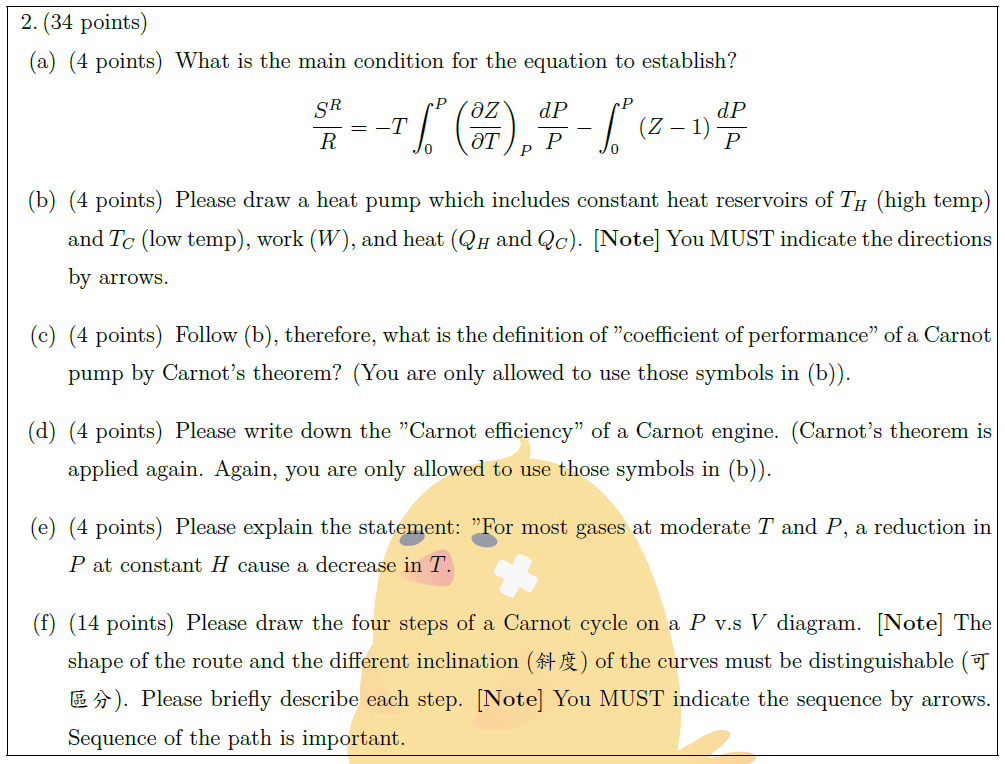
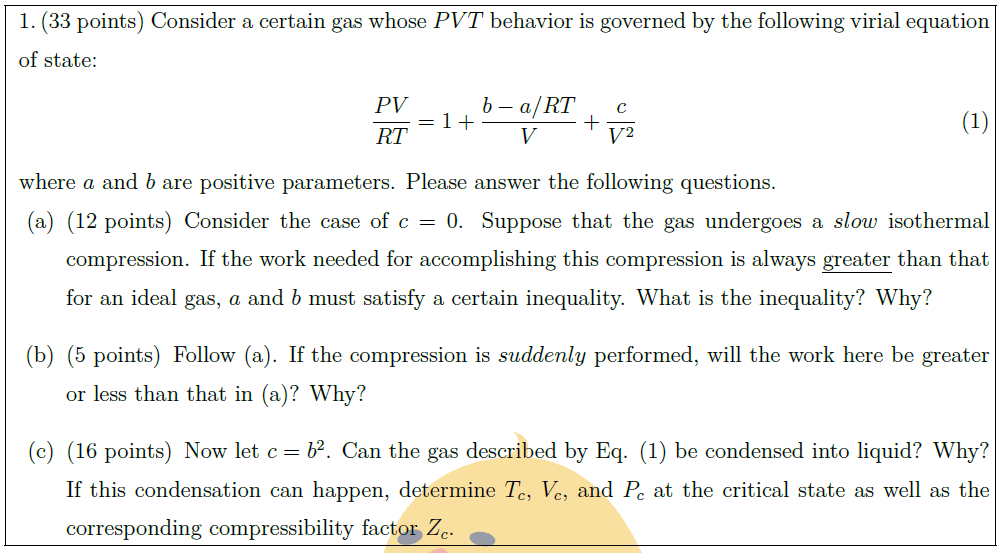
Solution:
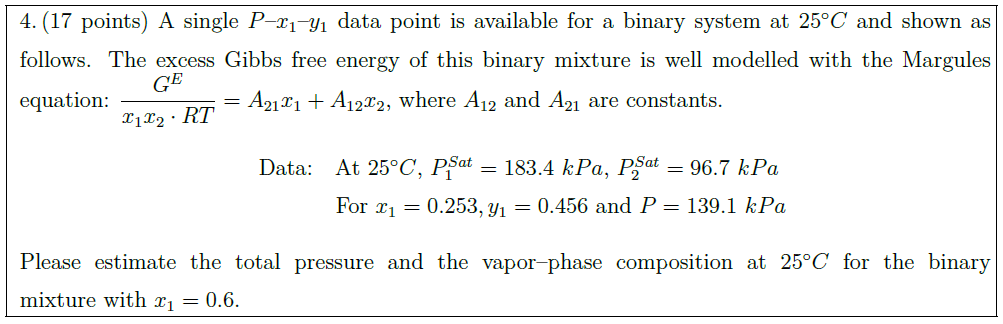
A single $P$–$x_1$–$y_1$ data point is available for a binary system at $25^\circ C$ and shown as follows. The excess Gibbs free energy of this binary mixture is well modelled with the Margules equation: $\displaystyle \frac{G^E}{x_1 x_2 \cdot RT} = A_{21} x_1 + A_{12} x_2$, where $A_{12}$ and $A_{21}$ are constants.\\
\\
\hspace*{10em}\begin{tabular}{cl}
Data: & At $25^\circ C$, $P_1^{Sat} = 183.4\ kPa$, $P_2^{Sat} = 96.7\ kPa$\\
& For $x_1 = 0.253, y_1 = 0.456$ and $P = 139.1\ kPa$
\end{tabular}\\
\\
Please estimate the total pressure and the vapor–phase composition at $25^\circ C$ for the binary mixture with $x_1 = 0.6$.