Solution:
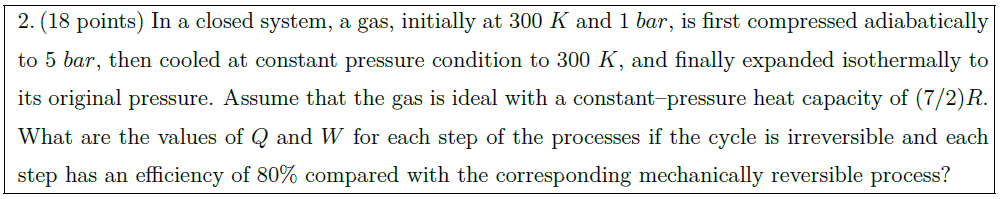
In a closed system, a gas, initially at $300\ K$ and $1\ bar$, is first compressed adiabatically to $5\ bar$, then cooled at constant pressure condition to $300\ K$, and finally expanded isothermally to its original pressure. Assume that the gas is ideal with a constant–pressure heat capacity of $(7 / 2) R$. What are the values of $Q$ and $W$ for each step of the processes if the cycle is irreversible and each step has an efficiency of $80 \%$ compared with the corresponding mechanically reversible process?