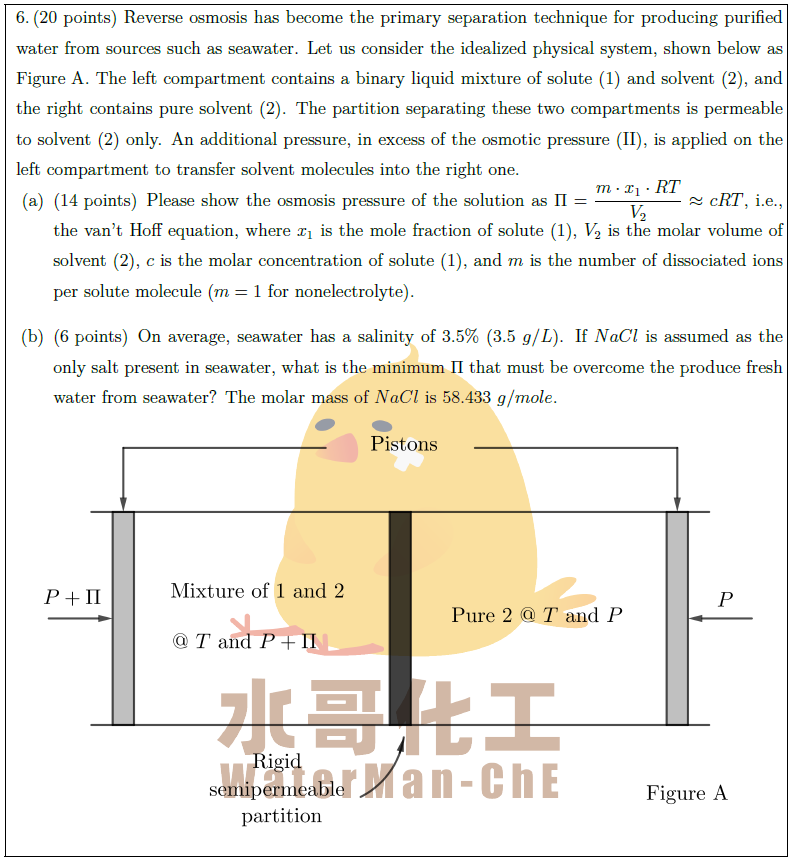
Solution:
A reversible compression of $1$ mole of an ideal gas in a cylinder device results in a pressure change from $0.5\ bar$ to $P_2$ and a temperature increasing from $300\ K$ to $750\ K$ by an adiabatic process. Please determine the $P_2$, work, $\Delta U$, and $\Delta H$.\vspace*{0.5em}\\
Assume: $\displaystyle \frac{C_P}{R} = 3.8 + 0.5 \times 10^{-3}\ T$ ($T$ is in unit of Kelvin, $K$.)