Solution:
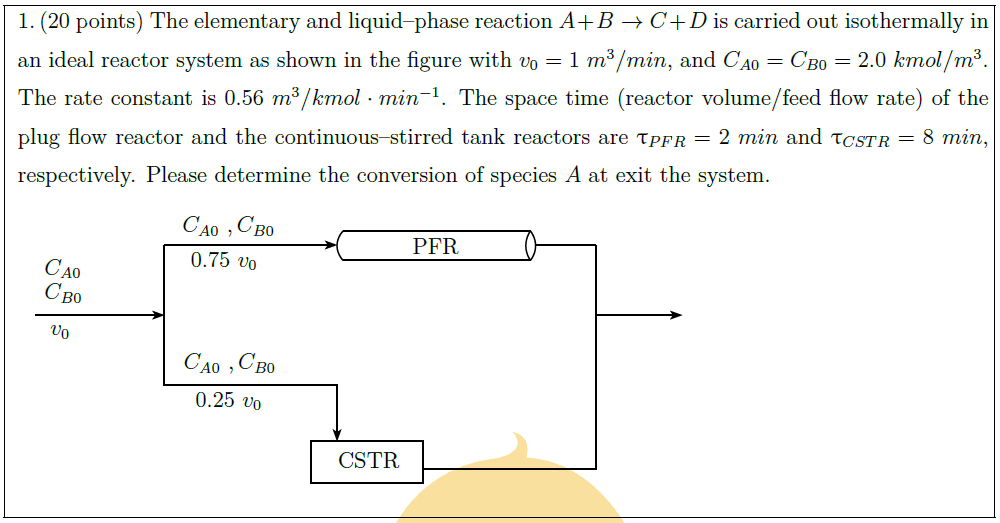
The elementary and liquid–phase reaction $A + B \to C + D$ is carried out isothermally in an ideal reactor system as shown in the figure with $v_0 = 1\ m^3/min$, and $C_{A0} = C_{B0} = 2.0\ kmol/m^3$. The rate constant is $0.56\ m^3/kmol \cdot min^{-1}$. The space time (reactor volume/feed flow rate) of the plug flow reactor and the continuous–stirred tank reactors are $\uptau_{PFR} = 2\ min$ and $\uptau_{CSTR} = 8\ min$, respectively. Please determine the conversion of species $A$ at exit the system.