Solution:
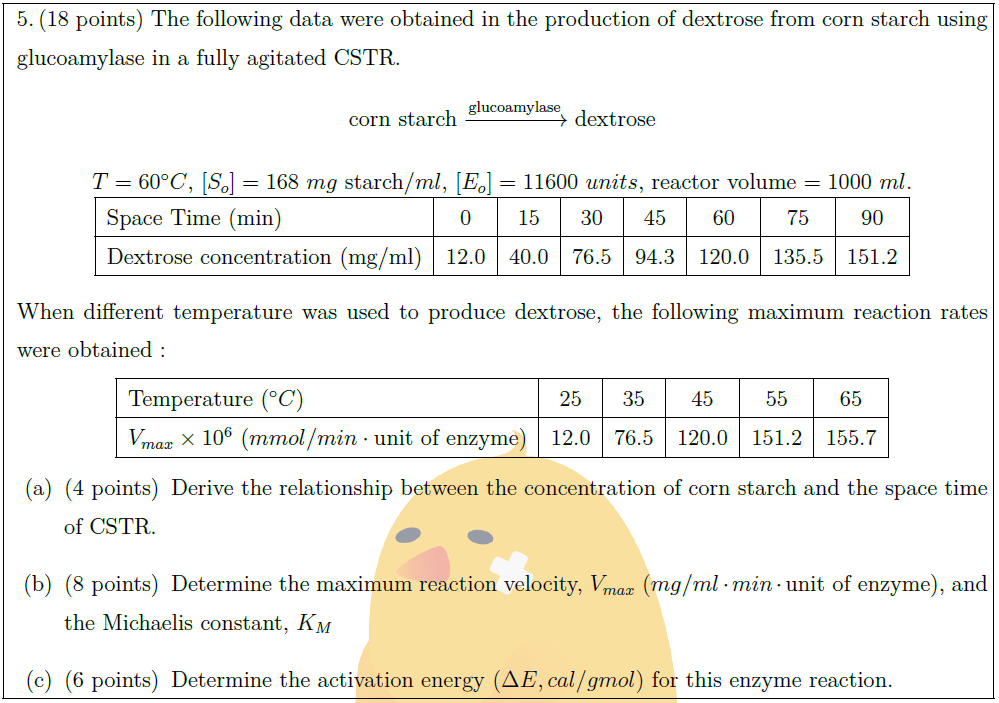
The following data were obtained in the production of dextrose from corn starch using glucoamylase in a fully agitated CSTR.
\begin{align*}
\mbox{corn starch} \xrightarrow{\mbox{\scriptsize glucoamylase}} \mbox{dextrose}
\end{align*}
\begin{center}
$T = 60^\circ C$, $[S_o] = 168\ mg\ \mbox{starch}/ml$, $[E_o] = 11600\ units$, reactor volume $= 1000\ ml$.\\
\begin{tabular}{|l|c|c|c|c|c|c|c|}
\hline
Space Time (min) & 0 & 15 & 30 & 45 & 60 & 75 & 90 \\
\hline
Dextrose concentration (mg/ml) & 12.0 & 40.0 & 76.5 & 94.3 & 120.0 & 135.5 & 151.2 \\
\hline
\end{tabular}
\end{center}
When different temperature was used to produce dextrose, the following maximum reaction rates were obtained :
\begin{center}
\begin{tabular}{|l|c|c|c|c|c|}
\hline
Temperature ($^\circ C$) & 25 & 35 & 45 & 55 & 65 \\
\hline
$V_{max} \times 10^6$ ($mmol/min \cdot \mbox{unit of enzyme}$) & 12.0 & 76.5 & 120.0 & 151.2 & 155.7 \\
\hline
\end{tabular}
\end{center}
\begin{parts}
\part [4] Derive the relationship between the concentration of corn starch and the space time of CSTR.
\part [8] Determine the maximum reaction velocity, $V_{max}$ ($mg/ml \cdot min \cdot \mbox{unit of enzyme}$), and the Michaelis constant, $K_M$
\part [6] Determine the activation energy ($\Delta E, cal/gmol$) for this enzyme reaction.
\end{parts}