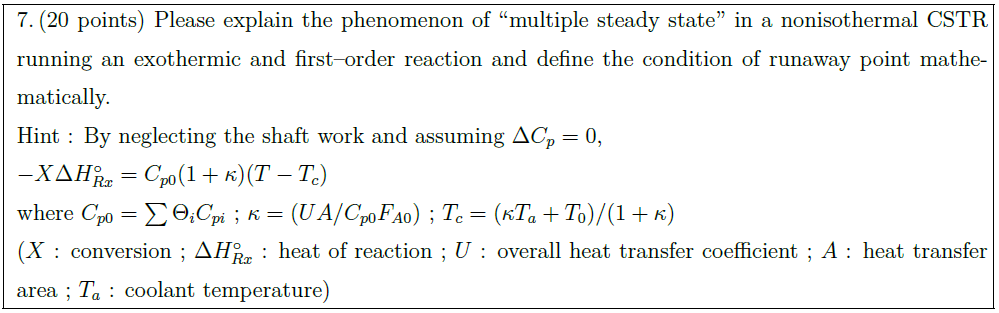
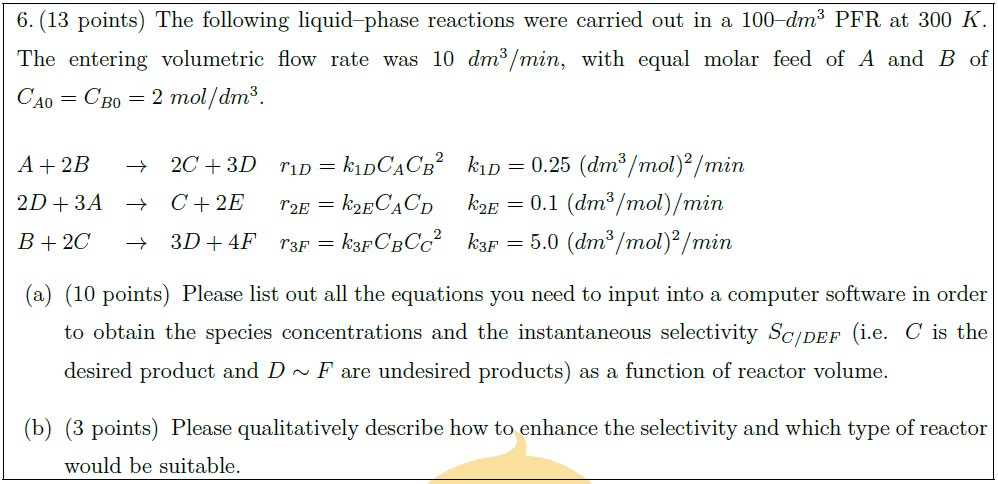
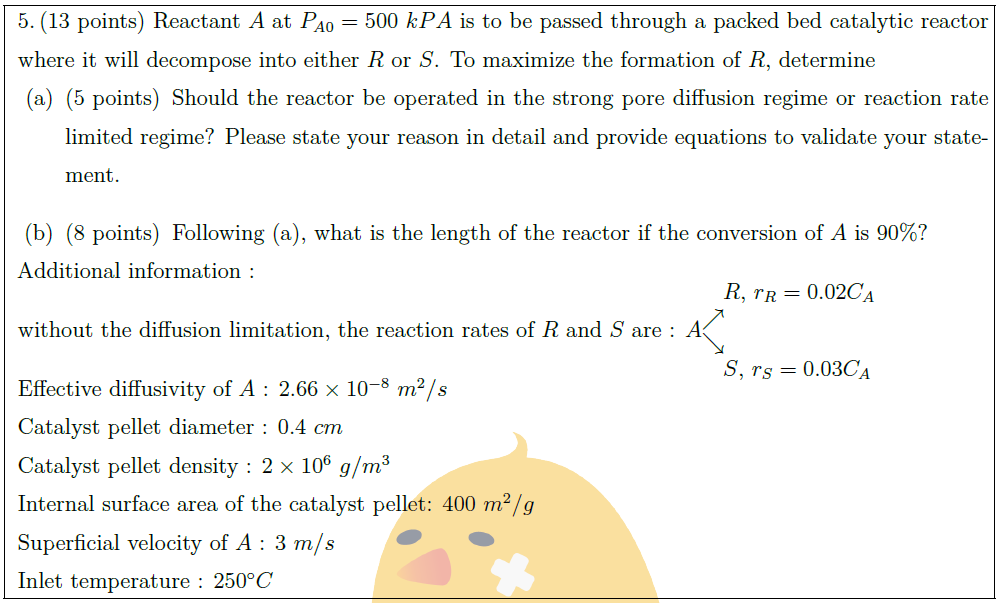
Solution:
Calculate the ratio of the volumes of a CSTR and a PFR ($V_{ST}/V_{PF}$) required to achieve a fraction of $0.99$ for the reactant $A$ with an identical feed rate for each reactor, if liquid–phase reaction $A \to$ products is
\begin{parts}
\part [5] first–order with respect to $A$ (please find the value of $V_{ST}/V_{PF}$?)
\part [7] second–order with respect to $A$ (please find the value of $V_{ST}/V_{PF}$?)
\end{parts}