Solution:
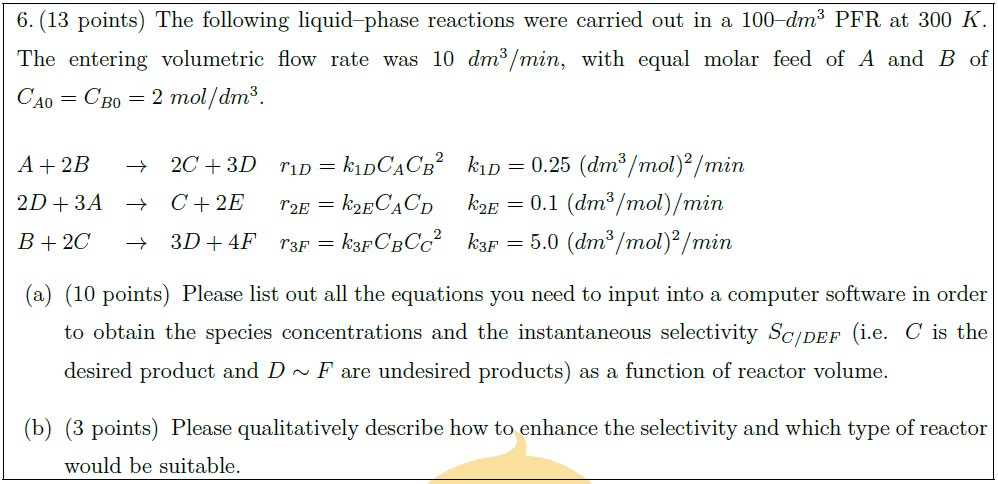
The following liquid–phase reactions were carried out in a 100–$dm^3$ PFR at $300\ K$. The entering volumetric flow rate was $10\ dm^3/min$, with equal molar feed of $A$ and $B$ of $C_{A0} = C_{B0} = 2\ mol/dm^3$.\\
\begin{tabular}{@{}lllll@{}}
$A + 2B$ & $\to$ & $2C + 3D$ & $r_{1D} = k_{1D} C_A {C_B}^2$ & $k_{1D} = 0.25\ (dm^3/mol)^2/min$\\
$2D + 3A$ & $\to$ & $C + 2E$ & $r_{2E} = k_{2E} C_A C_D$ & $k_{2E} = 0.1\ (dm^3/mol)/min$\\
$B + 2C$ & $\to$ & $3D + 4F$ & $r_{3F} = k_{3F} C_B {C_C}^2$ & $k_{3F} = 5.0\ (dm^3/mol)^2/min$
\end{tabular}\\
\begin{parts}
\part [10] Please list out all the equations you need to input into a computer software in order to obtain the species concentrations and the instantaneous selectivity $S_{C/DEF}$ (i.e. $C$ is the desired product and $D \sim F$ are undesired products) as a function of reactor volume.
\part [3] Please qualitatively describe how to enhance the selectivity and which type of reactor would be suitable.
\end{parts}