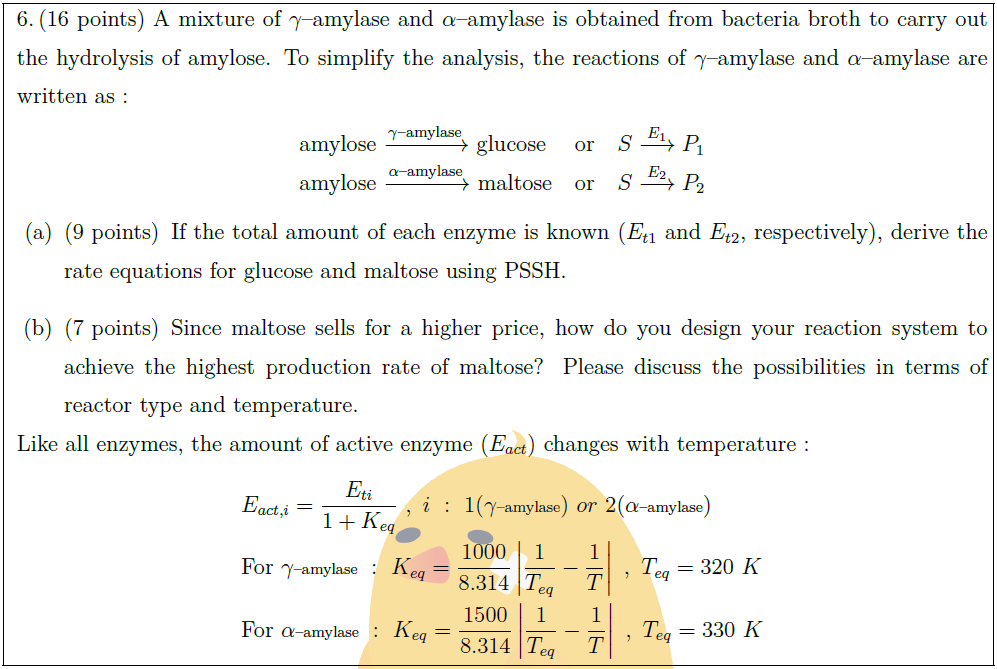
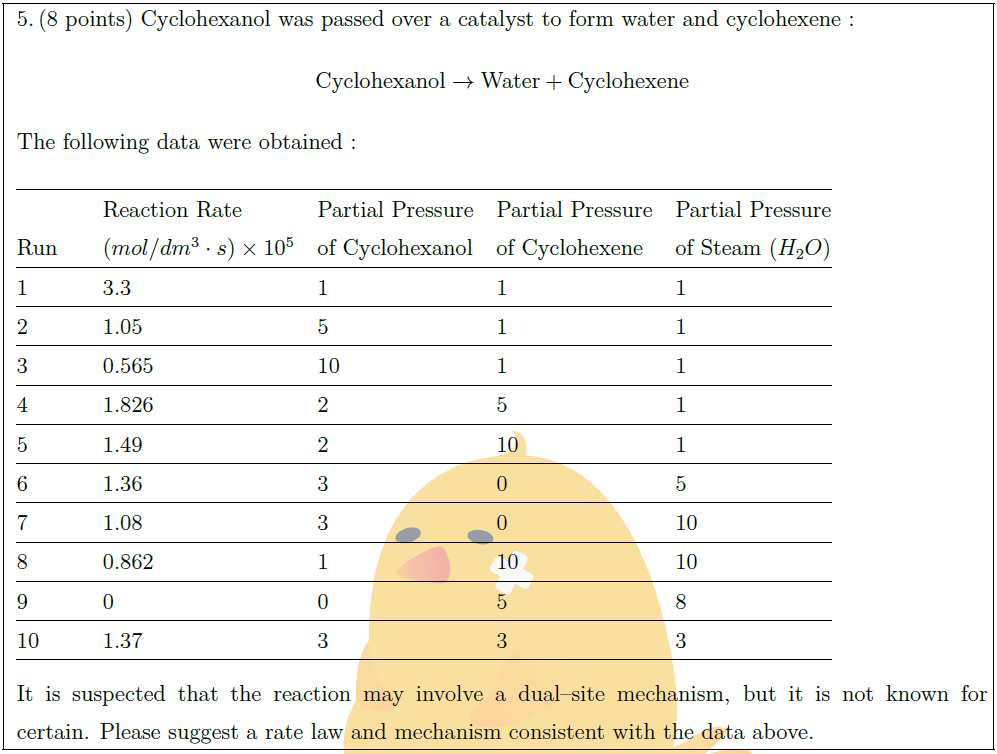
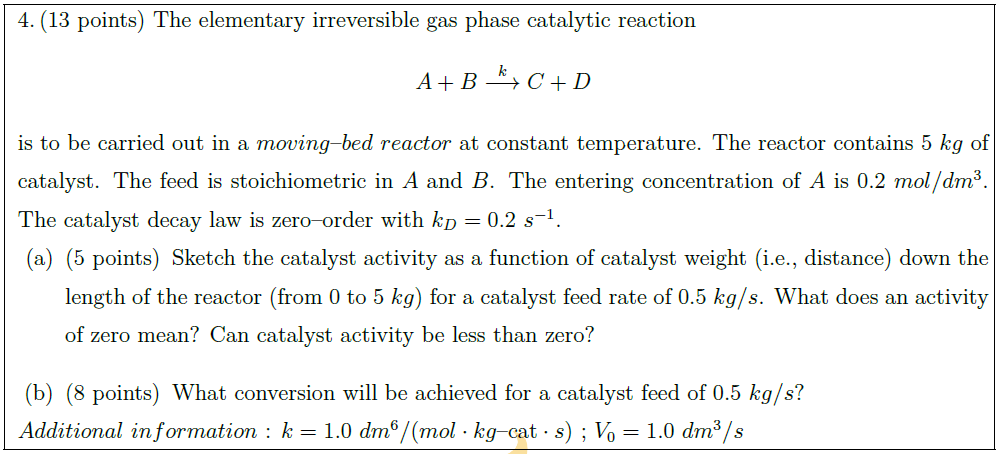
Solution:
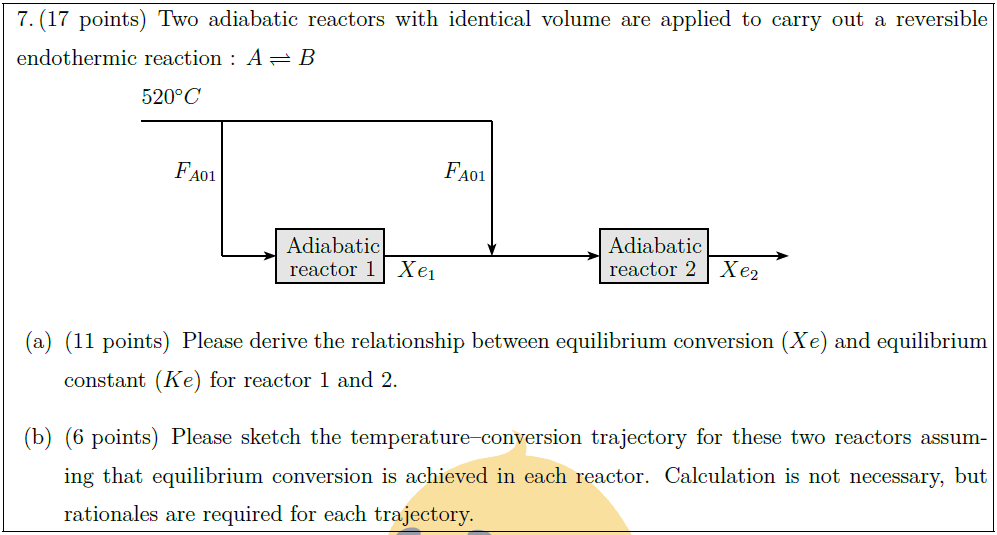
Two adiabatic reactors with identical volume are applied to carry out a reversible endothermic reaction : $\displaystyle A \rightleftharpoons B$
\begin{parts}
\part [11] Please derive the relationship between equilibrium conversion ($Xe$) and equilibrium constant ($Ke$) for reactor 1 and 2.
\part [6] Please sketch the temperature–conversion trajectory for these two reactors assuming that equilibrium conversion is achieved in each reactor. Calculation is not necessary, but rationales are required for each trajectory.
\end{parts}