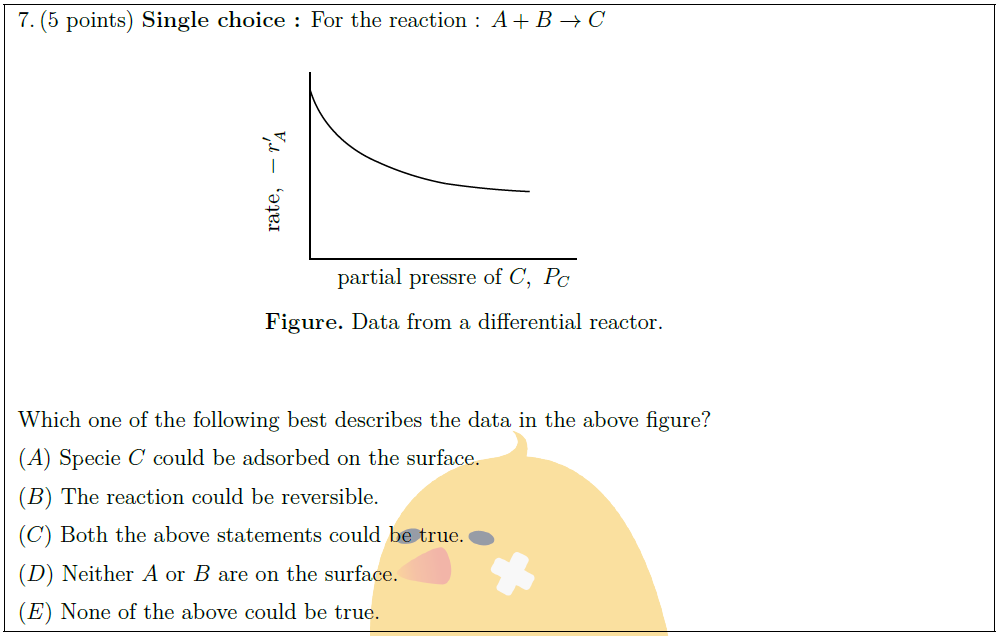
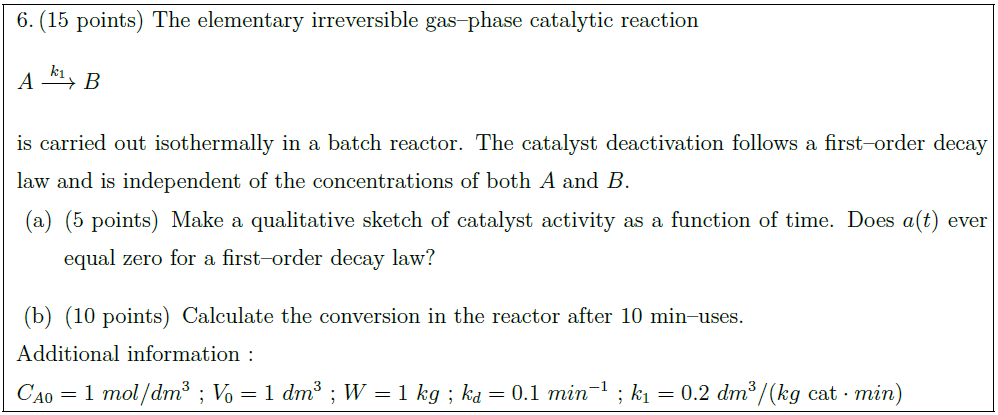
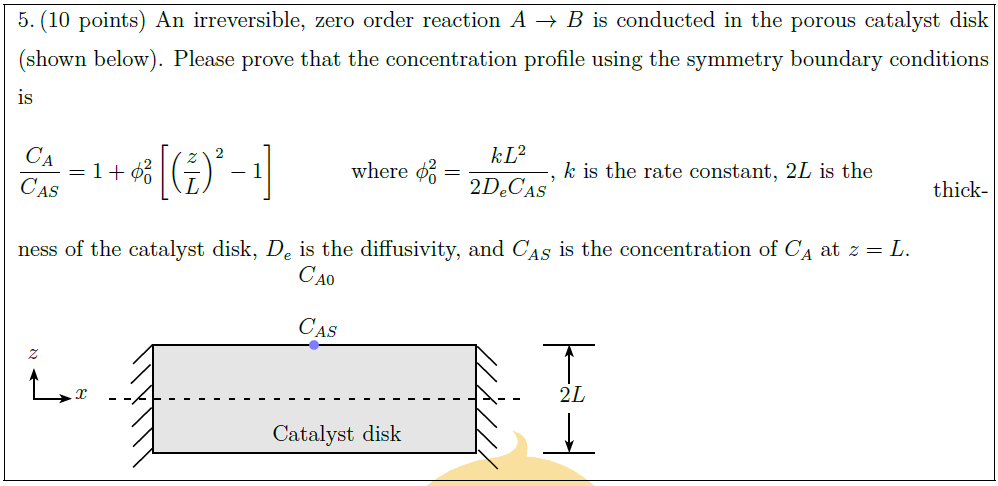
Solution:
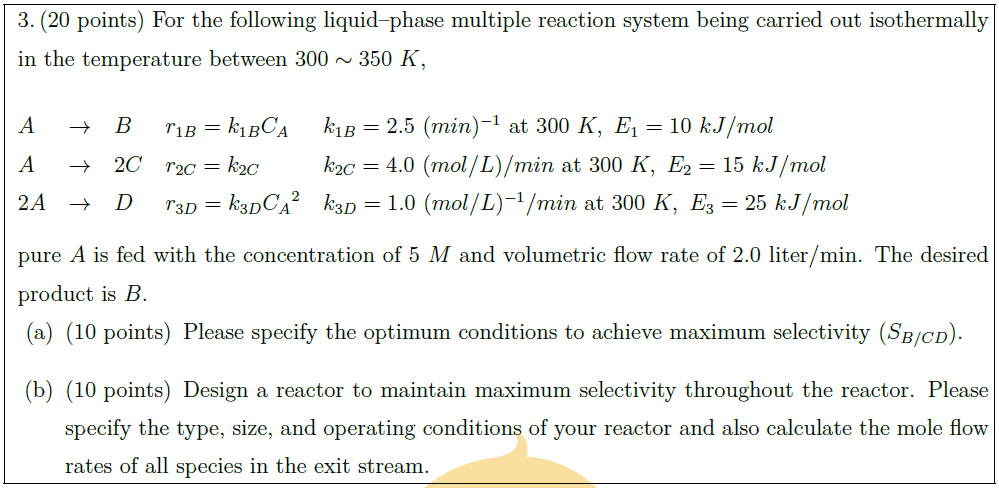
For the following liquid–phase multiple reaction system being carried out isothermally in the temperature between $300 \sim 350\ K$,\\
\begin{tabular}{@{}lllll@{}}
$A$ & $\to$ & $B$ & $r_{1B} = k_{1B} C_A$ & $k_{1B} = 2.5\ (min)^{-1}\ \mbox{at}\ 300\ K,\ E_1 = 10\ kJ/mol$\\
$A$ & $\to$ & $2C$ & $r_{2C} = k_{2C}$ & $k_{2C} = 4.0\ (mol/L)/min\ \mbox{at}\ 300\ K,\ E_2 = 15\ kJ/mol$\\
$2A$ & $\to$ & $D$ & $r_{3D} = k_{3D}{C_A}^2$ & $k_{3D} = 1.0\ (mol/L)^{-1}/min\ \mbox{at}\ 300\ K,\ E_3 = 25\ kJ/mol$
\end{tabular}\\
pure $A$ is fed with the concentration of $5\ M$ and volumetric flow rate of $2.0$ liter/min. The desired product is $B$.
\begin{parts}
\part [10] Please specify the optimum conditions to achieve maximum selectivity ($S_{B/CD}$).
\part [10] Design a reactor to maintain maximum selectivity throughout the reactor. Please specify the type, size, and operating conditions of your reactor and also calculate the mole flow rates of all species in the exit stream.
\end{parts}