Solution:
\begin{parts}
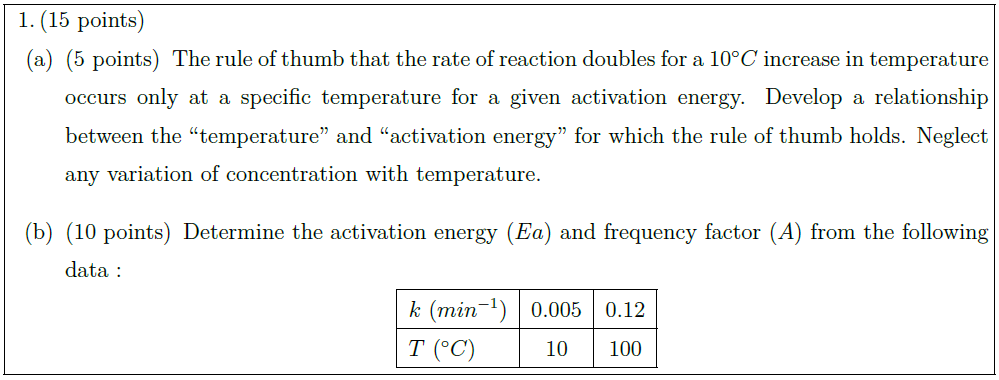
\part [5] The rule of thumb that the rate of reaction doubles for a $10^\circ C$ increase in temperature occurs only at a specific temperature for a given activation energy. Develop a relationship between the “temperature” and “activation energy” for which the rule of thumb holds. Neglect any variation of concentration with temperature.
\part [10] Determine the activation energy ($Ea$) and frequency factor ($A$) from the following data :
\begin{center}
\begin{tabular}{|l|c|c|}
\hline
$k\ (min^{-1})$ & 0.005 & 0.12\\
\hline
$T\ (^\circ C)$ & 10 & 100\\
\hline
\end{tabular}
\end{center}
\end{parts}