Solution:
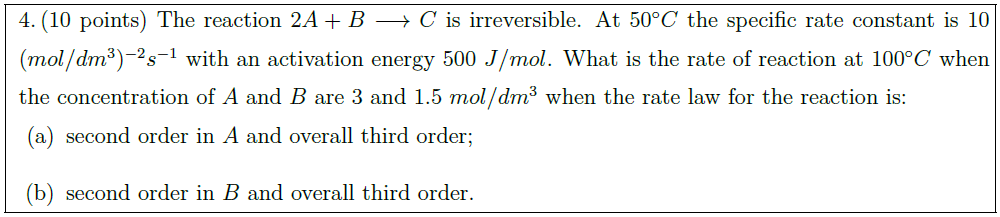
The reaction $2A + B \longrightarrow C$ is irreversible. At $50^\circ C$ the specific rate constant is 10 $(mol / dm^3)^{-2} s^{-1}$ with an activation energy $500\ J/ mol$. What is the rate of reaction at $100^\circ C$ when the concentration of $A$ and $B$ are $3$ and $1.5\ mol / dm^3$ when the rate law for the reaction is:
\begin{parts}
\part second order in $A$ and overall third order;
\part second order in $B$ and overall third order.
\end{parts}